Kit for screening and checking glucose-6-phosphate dehydrogenase (G6PD) deficiency of neonates and preparation method for kit
A hexaphosphate dehydrogenase and glucose technology, which is applied to a kit for screening neonatal glucose hexaphosphate dehydrogenase deficiency and its preparation field, can solve the problems of high false positive rate and low accuracy rate of blood samples, and achieves The effect of reducing false positive rate, high accuracy and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The components of the simultaneous detection G6PD assay kit (fluorescence ratio method) of the present invention include:
[0051] (1) G6P substrate reagent dry powder: made of G-6-P and NADP and freeze-dried;
[0052] (2) 6PG substrate reagent dry powder: made of 6-PG and NADP and freeze-dried;
[0053] (3) Copper reagent: made of copper sulfate, potassium sodium tartrate and sodium carbonate;
[0054] (4) G6PD filter paper dry blood spot quality control, C1 positive, C2 negative: use anticoagulated sheep whole blood red blood cells to prepare filter paper dry blood spot;
[0055] (5) Reagent for substrate reconstitution: made with MgCl2, Gly-Gly (diglycine) and Tris-Hcl.
[0056] (6) Disc quality control: citrate anticoagulated sheep whole blood in a low-speed centrifuge at 3000rmp, and centrifuge for 10-15min to separate plasma and red blood cells. Adjust the hematocrit according to the ratio (red blood cell: plasma = 1.2: 1.0); add G6PD and 6PGD pure raw material...
Embodiment 2
[0068] Embodiment 2 The usage method of kit of the present invention
[0069] The specific operation of the neonatal G6PD screening kit (fluorescence ratio method) prepared in Example 1 above is as follows:
[0070] Reagent preparation:
[0071] Substrate redissolution: G6P substrate reagent dry powder, 6PG substrate reagent dry powder respectively add 16ml substrate redissolution reagent, mix well, let stand for 20 minutes;
[0072] Cooling of the copper reagent: the reagent must be cooled in a refrigerator at 4°C before use.
[0073] Check operation:
[0074] Adding samples: Punch out two samples with a diameter of about 3mm (1 / 8 inch) from the sample filter paper dry blood sheet with punching forceps (the paper sheet must be soaked with blood), and add the blanks of G6PD and 6PGD respectively in turn In the microwell, each hole corresponds to one piece. Control the consistency of punching to make the paper as consistent as possible.
[0075] Add substrate: add 150 μl o...
Embodiment 3
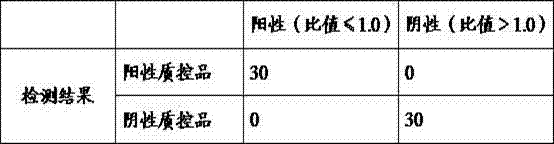
[0080] Example 3 Analysis performance evaluation index of the kit of the present invention
[0081] The performance evaluation indexes of the newborn screening assay G6PD kit (fluorescence ratio method) prepared in the above Example 1 are as follows:
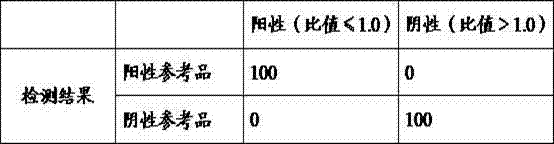
[0082] Sensitivity: determine 1 set of positive reference products established by the company's laboratory. Positive reference products are samples from patients with G6PD deficiency determined by clinical testing, consisting of 100 samples.
[0083] Conformity rate of positive reference products (%) = measured number of positive reference products / 10*100%.
[0084] Table 2 Sensitivity test of kit of the present invention
[0085] Positive (ratio ≤ 1.0) Negative (ratio > 1.0) Test results 100 0
[0086] Continuing through the above table, the positive coincidence rate is 100%.
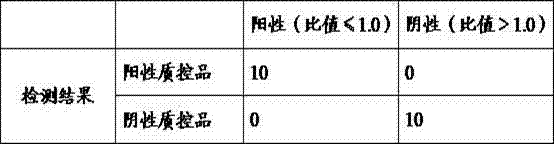
[0087] specificity:
[0088] Determination of conformity rate of negative reference products: 1 set of negative reference...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com