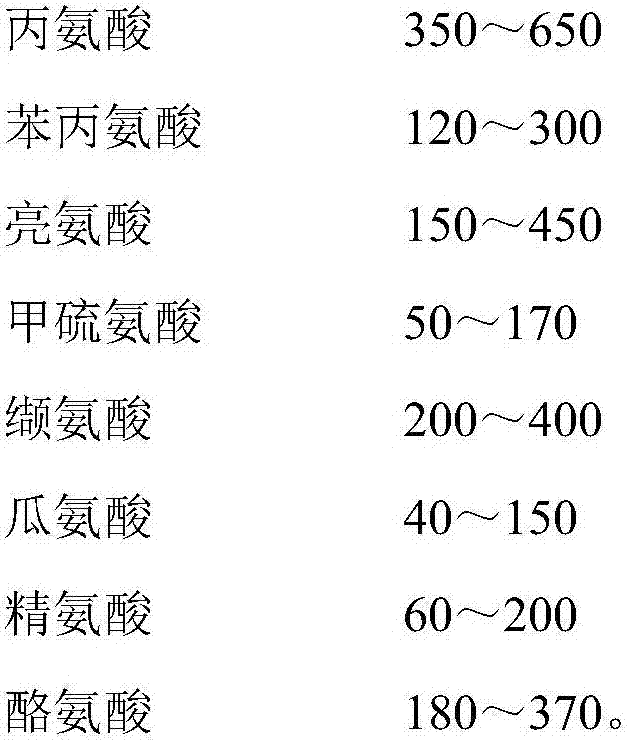
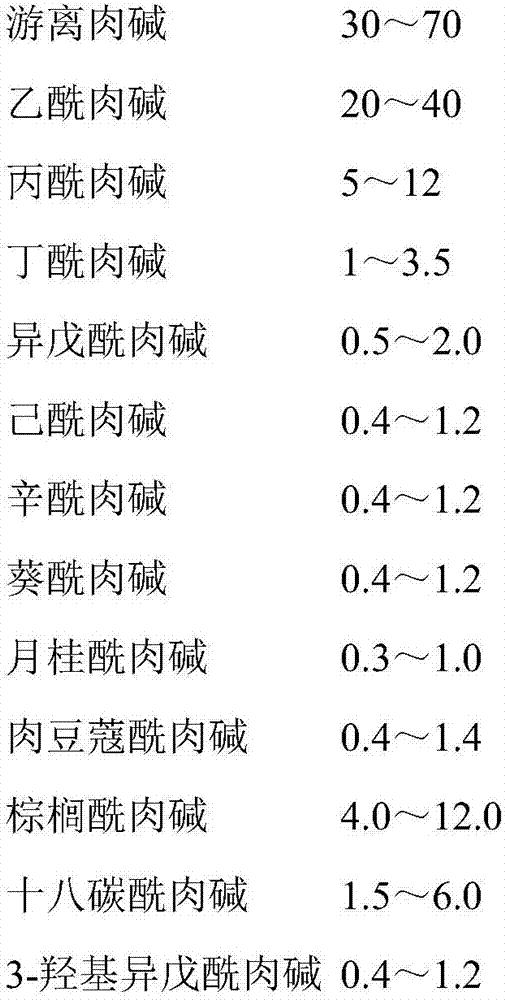
Amino acid and acyl carnitine dry blood spot control material and preparing method thereof
A technology of acylcarnitine and amino acid, which is applied in the field of amino acid and acylcarnitine dried blood spot quality control products and its preparation, and can solve the problems of high product price, no stable source, and different characteristics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0037] A method for preparing the above-mentioned amino acid and acylcarnitine dried blood spot quality control product, comprising the steps of:
[0038] 1) Preparation of erythrocyte fluid: collect healthy human whole blood, separate red blood cells, adjust the hematocrit to a predetermined value with normal saline, obtain erythrocyte fluid, and measure the background value of each amino acid and acylcarnitine;
[0039] 2) Preparation of standard solution: using an aqueous solvent to dissolve amino acids and / or water-soluble acylcarnitines; using alcohol solvents to dissolve fat-soluble acylcarnitines to obtain amino acid standard solutions and / or acylcarnitine standard solutions;
[0040] 3) Preparation of blood spot quality control: Add amino acid standard solution and / or acylcarnitine standard solution to the set value to the above red blood cell solution to obtain a working solution; take the above working solution dropwise on the carrier, dry to obtain amino acid and acy...
Embodiment 1
[0057] 1) Separation of red blood cells:
[0058] Collect healthy human heparin anticoagulated whole blood, requiring no obvious hemolysis, jaundice, fat or pollution, centrifuge at a speed of 3000r / min for 5min, separate the supernatant and white membrane after centrifugation, add about the same amount of normal saline as the amount of red blood cells , use a mixer to mix well for 5-7min, centrifuge at 3000r / min for 5min, separate the supernatant and buffy membrane, repeat this step 3 times to obtain red blood cells. Normal saline was used to adjust the hematocrit to 50% to obtain erythrocyte fluid, and the background concentrations of amino acids and acylcarnitine in the erythrocyte fluid were determined.
[0059] 2) Preparation of amino acids and acylcarnitines:
[0060] Use physiological saline to dissolve 8 amino acid standards including alanine Ala, phenylalanine Phe, leucine Lue, methionine Met, valine Val, citrulline Cit, arginine Arg, and tyrosine Tyr product, to ob...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com