Alpha-thalassemia screening kit and application thereof in prenatal screening
A technology for thalassemia and prenatal screening, applied in the direction of microbial determination/examination, biochemical equipment and methods, etc., can solve the problems of invasive inspection, invasive operation, fetal impact, etc., achieving high accuracy and reducing Birth rates, effects of filling technological gaps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
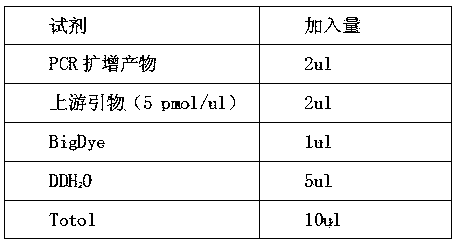
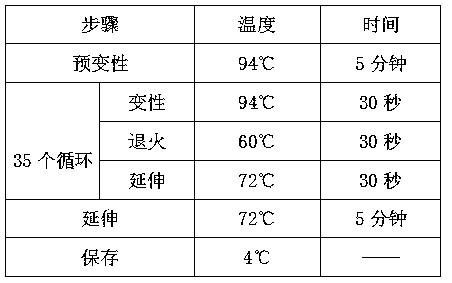
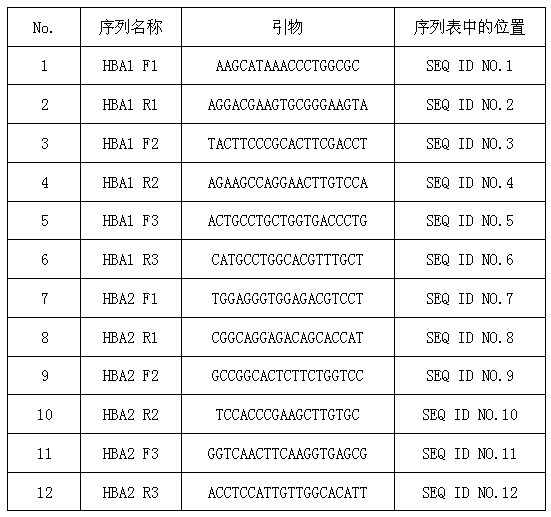
[0027] 10 mL of peripheral blood from 100 pregnant women were collected from 3 hospitals, and prenatal screening for α-thalassemia was carried out according to the following steps.
[0028] (1) Extraction of cell-free fetal DNA from peripheral blood of pregnant women;
[0029] ① Aseptically collect 10 mL of whole blood in 15 mL vacuum EDTA anticoagulant blood collection tubes to avoid hemolysis.
[0030] ②Centrifuge at 800g in a low-temperature centrifuge at 4°C for 10 minutes, and absorb the supernatant.
[0031] ③ Take the supernatant (about 4-5mL of plasma) and transfer it to a new 15 mL polypropylene test tube, centrifuge at 1600g, 4°C for 10 minutes.
[0032] ④ Take 1 mL of the supernatant (2.0 mL new polypropylene test tube), add 1 / 10 volume of proteinase K buffer (10X), pulse shake and mix for 30 seconds; then add proteinase K (proteinase K, 30∪ / mL, no DNase and RNase) to a final concentration of 0.1-1 mg / mL, pulse-shake and mix for 30 seconds.
[0033] ⑤Water at 60°...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com