A method and kit for detecting and evaluating anti-HBV drug activity
A drug activity and drug technology, applied in the field of detection and evaluation of anti-HBV drug activity and kits, can solve the problems of inaccurate and comprehensive test results, achieve reasonable primer design, high gene expression level, and increased detection sensitivity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
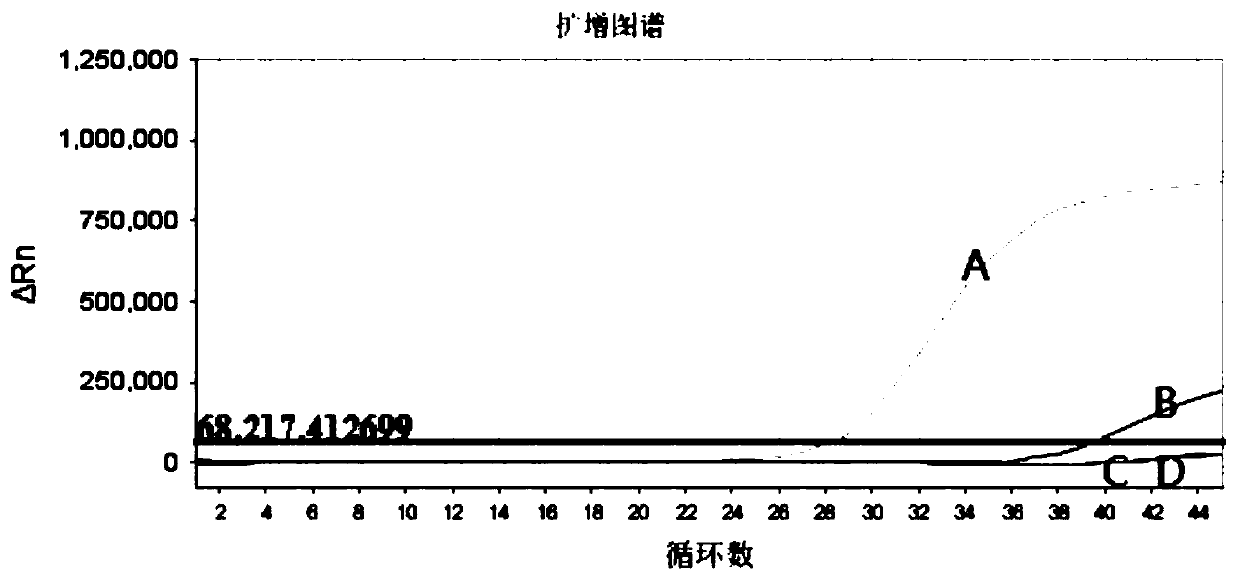
Image
Examples
Embodiment 1
[0048] Embodiment 1, real-time fluorescent quantitative PCR kit
[0049] 1. Kit composition
[0050] The kit contains: upstream primers, downstream primers, Taqman probes and reverse transcription primers; where:
[0051] The sequence of the upstream primer (F1) is:
[0052] 5'-CATGCAACTTTTTTCACCTCTGC-3' (as shown in sequence listing sequence 1);
[0053] The sequence of the downstream primer (R1) is:
[0054] 5'-GAACTGCCAATGATCGTACG-3' (as shown in sequence listing sequence 2);
[0055] The sequence of the Taqman probe (FP) is:
[0056] 5'-ATCTCATGTTCATGTCCTACTGTTCA-3' (as shown in Sequence Listing Sequence 3); its 5' end is marked with fluorescence, and its 3' end is marked with fluorescence;
[0057] The sequence of the reverse transcription primer (RT) is:
[0058] 5'-GAACTGCCAATGATCGTACGTTTTTTTTTTTTTTTTTTTTTTTTTTTVN-3' (shown in sequence 4).
[0059] The composition and content of each reagent in the kit are as follows:
[0060] Quality control: HBV pgRNA negative...
Embodiment 2
[0069] Embodiment 2, the real-time fluorescent quantitative PCR detection of HBV pgRNA content in the sample
[0070] 1. Sample extraction
[0071] Take out the HBV pgRNA internal standard quality control product, proteinase K, sedimentation aid and seven quality control products stored at -20°C, melt at room temperature, shake for 15 seconds, and centrifuge briefly.
[0072] Shake the serum to be tested for 15s and mix thoroughly. Take seven quality controls (pgRNA negative quality control, pgRNA critical positive quality control, pgRNA positive quality control and pgRNA quantitative standard (Ⅰ, Ⅱ, Ⅲ, Ⅳ)) and 200μL to 1.5mL of the serum submitted for testing In a centrifuge tube, add 300 μL of virus lysate, 5 μL of HBV pgRNA internal standard quality control, 10 μL of proteinase K, 4 μL of sedimentation aid and 20 μL of magnetic beads (mix by inversion before each pipetting), and mix gently by inversion at room temperature. Homogenize for 10 minutes to fully combine the ma...
Embodiment 3
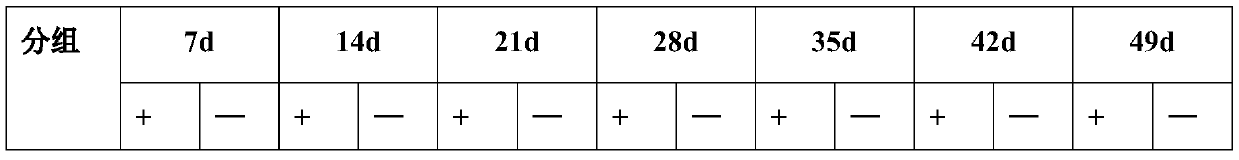
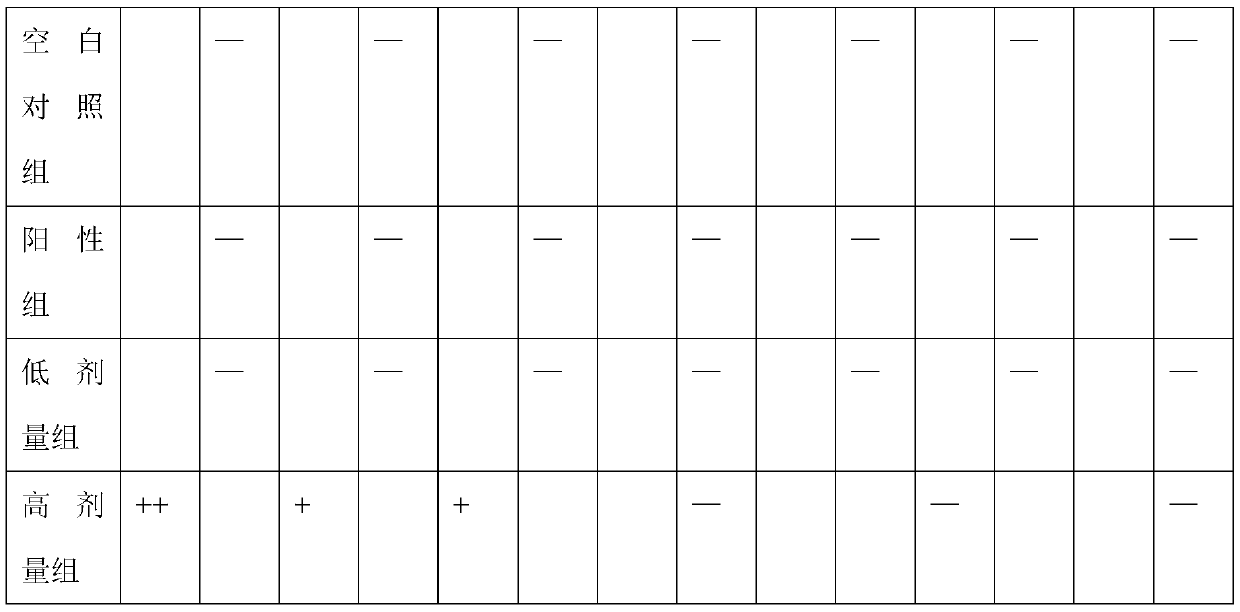
[0093] Embodiment 3, detection experiment of anti-HBV drug activity in mice
[0094] 1. Experimental materials
[0095] (1) Main reagents: Hepatitis B drug M, FQ-PCR kit (real-time fluorescence quantitative PCR kit), HBs Ag (hepatitis B surface antigen) ELISA kit.
[0096] (2) Main instruments: automatic microplate reader, FQ real-time fluorescent quantitative PCR instrument
[0097] (3) Animals: female HBV transgenic mice, provided by the Experimental Animal Center of Peking University. The female HBV transgenic mice were developed by microinjecting 1.5 copies of HBV DNA into Balb / c mouse embryos. High levels of HBsAg and HBV DNA could be detected in the serum of the female HBV transgenic mice. In this experiment, 10-week-old adult mice were used.
[0098] 2. Experimental method
[0099] The transgenic mice were randomly divided into 3 groups, namely the positive group, the low-dose drug group and the high-dose drug group, and a blank control group (ie, female non-transg...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com