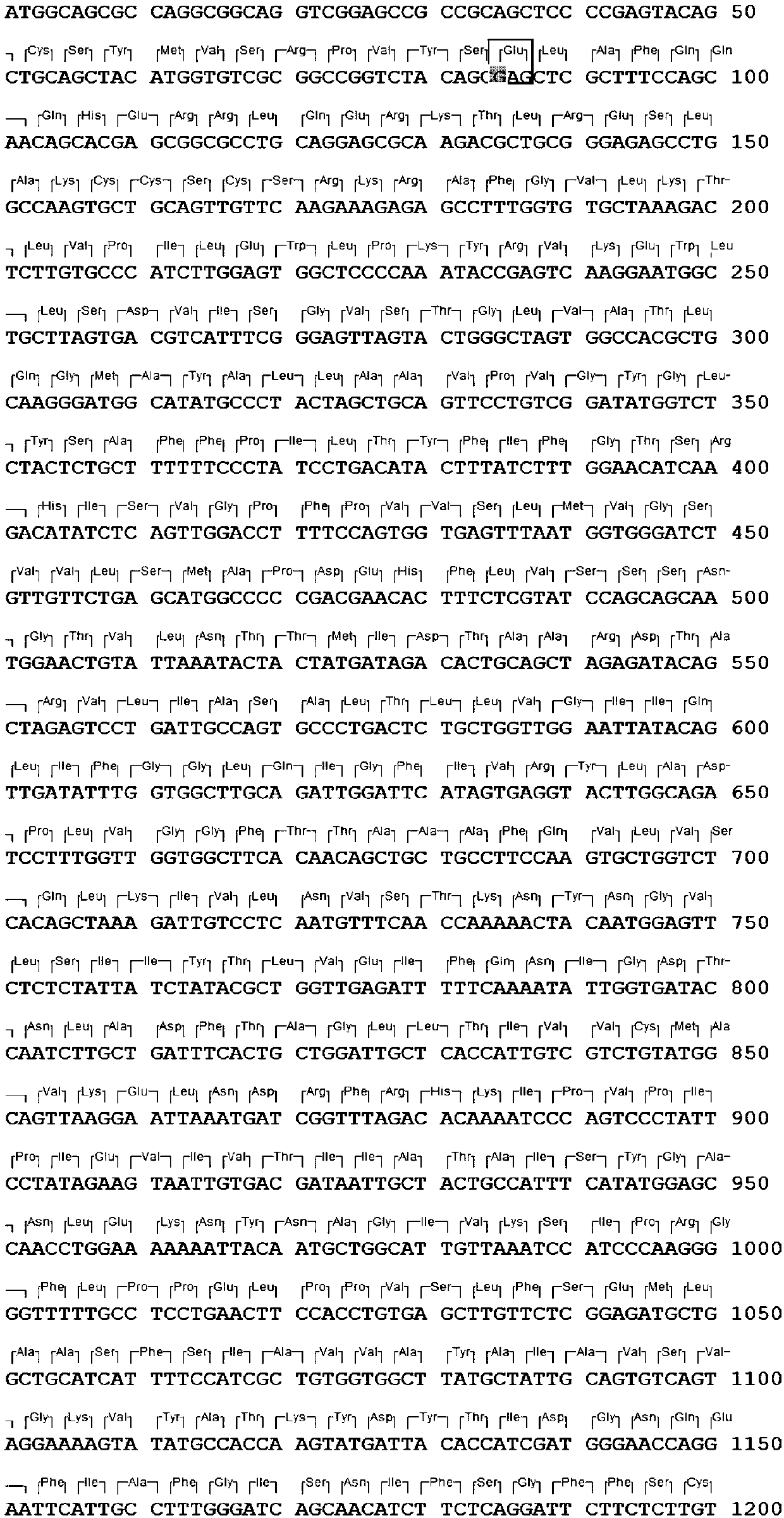
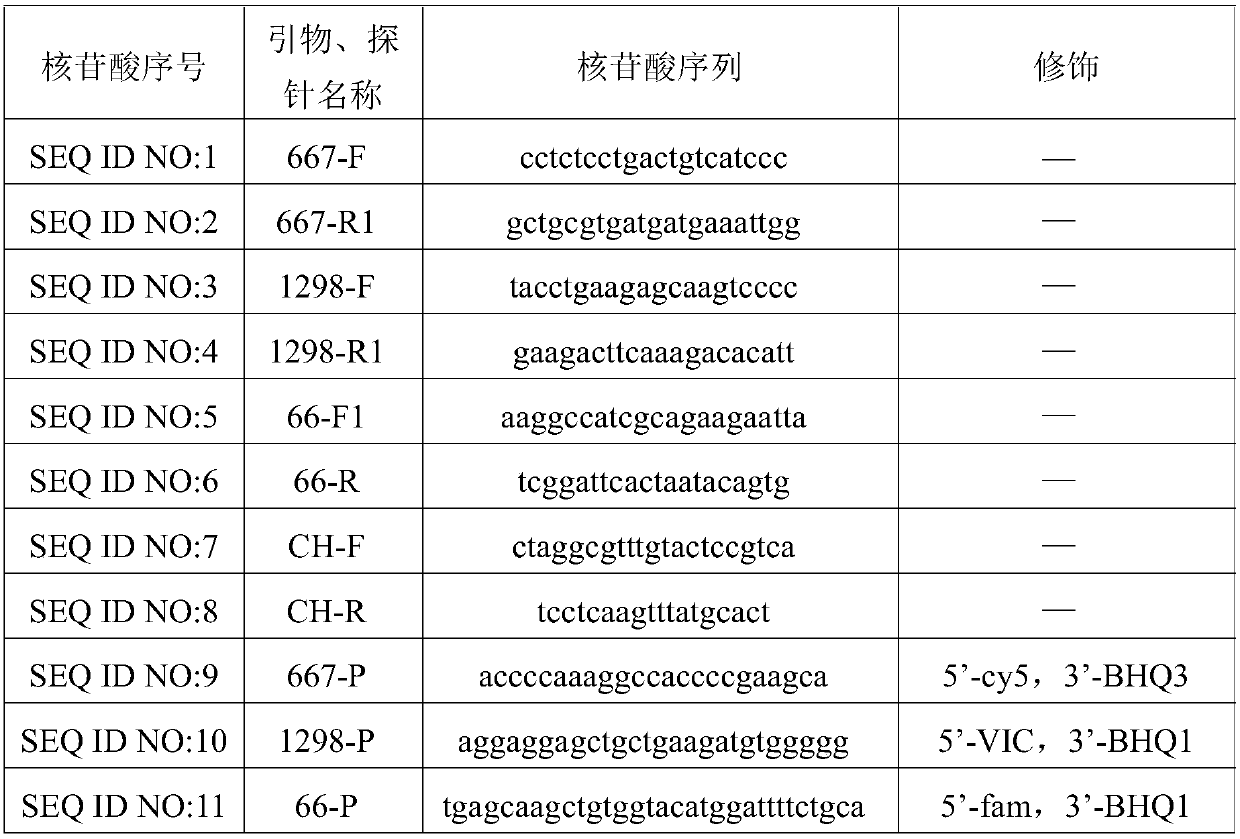
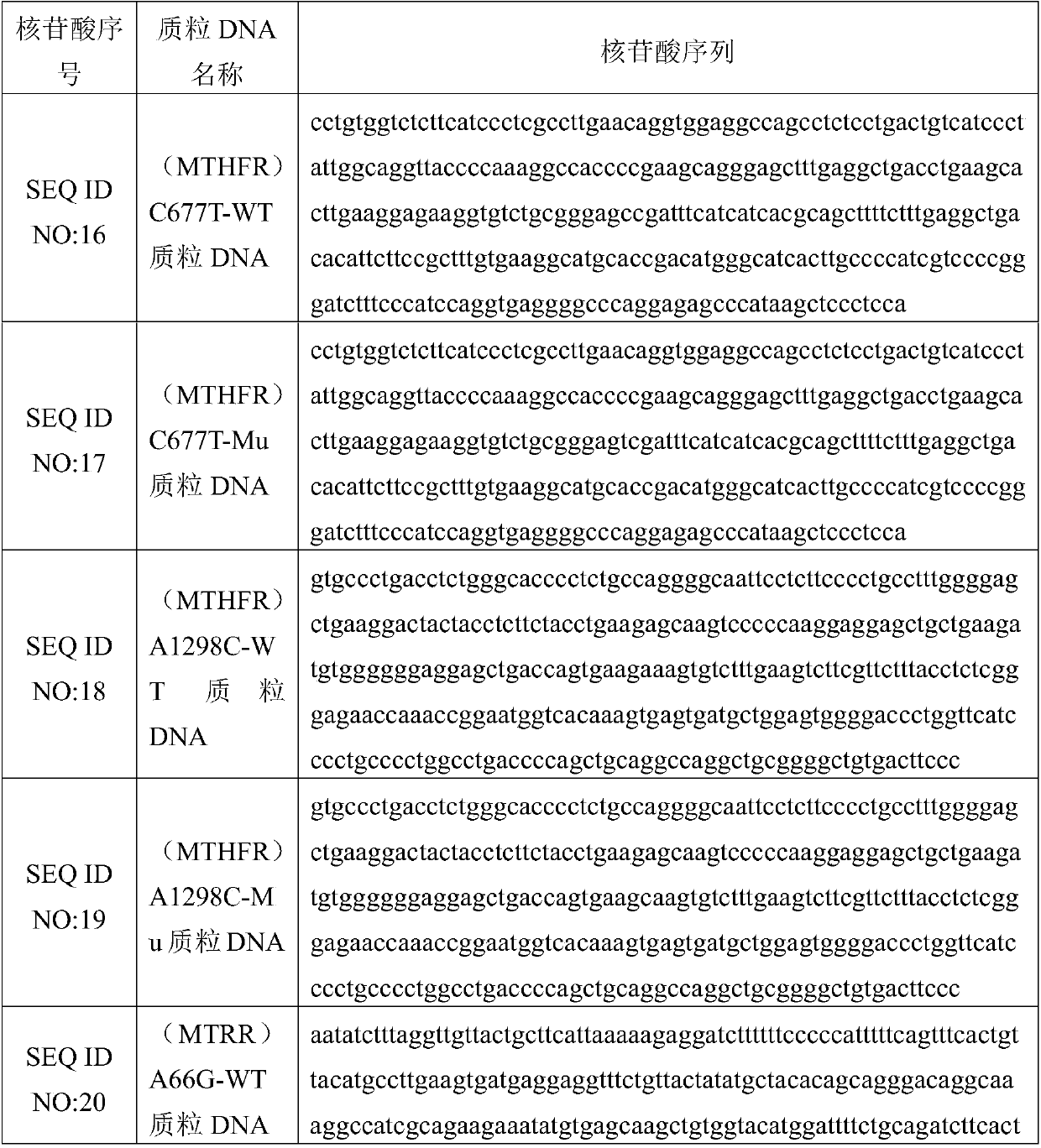
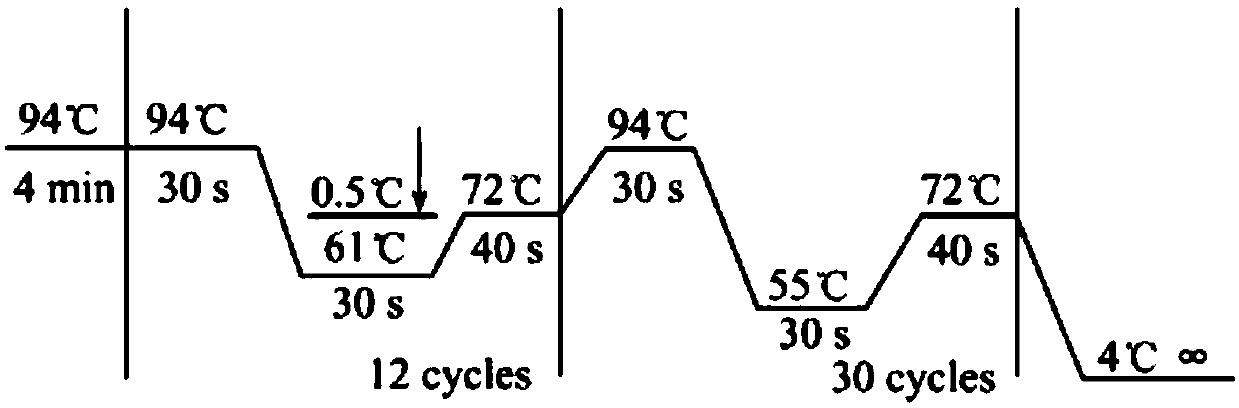
Patents
Literature
35results about How to "Reduce birth rate" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
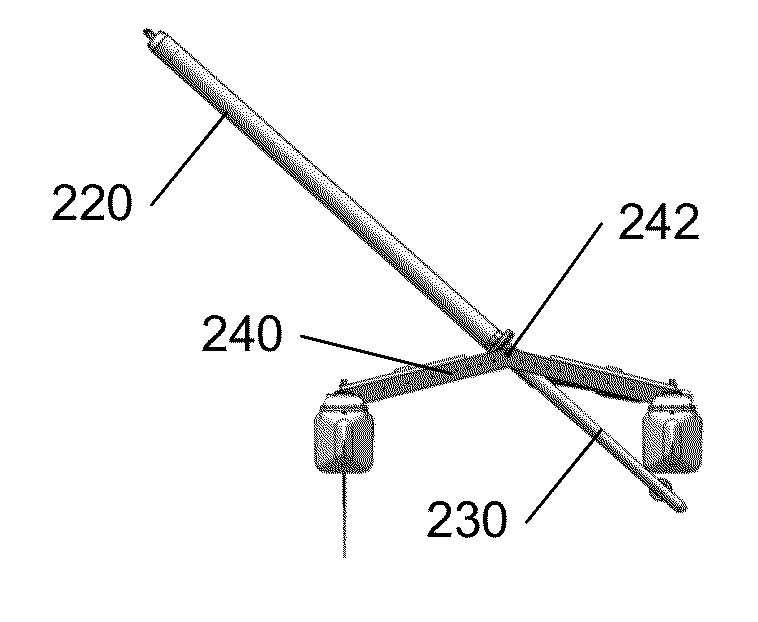
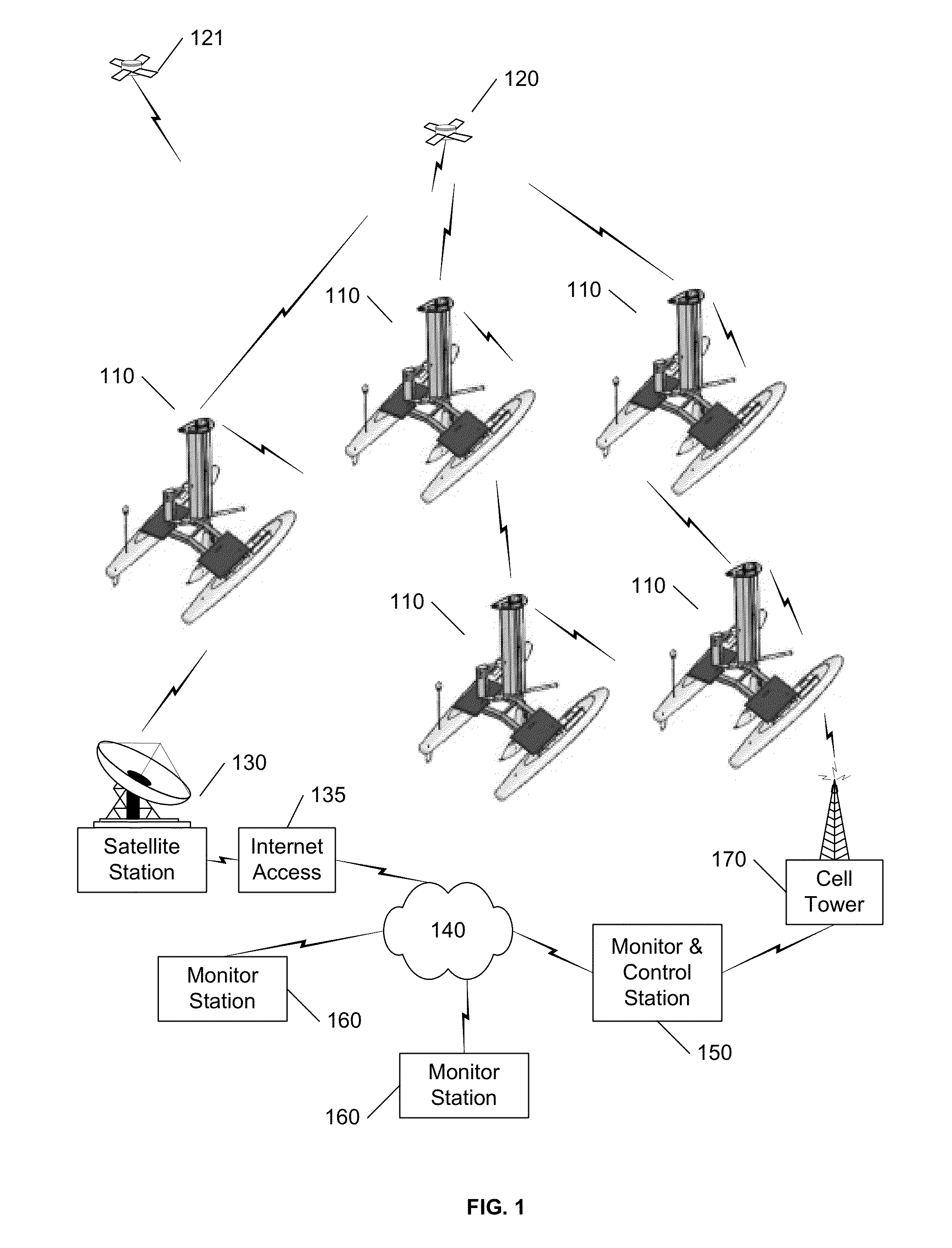
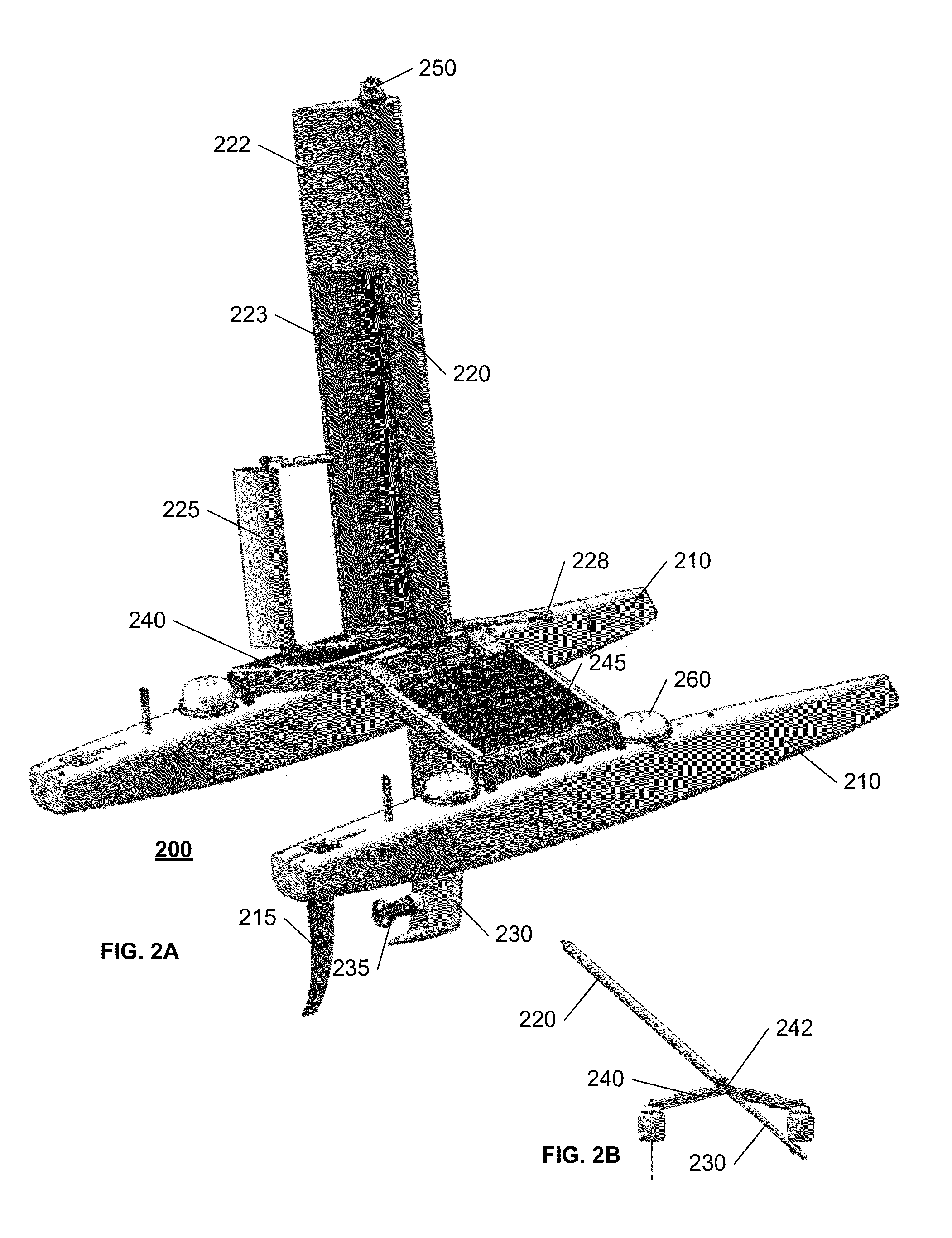
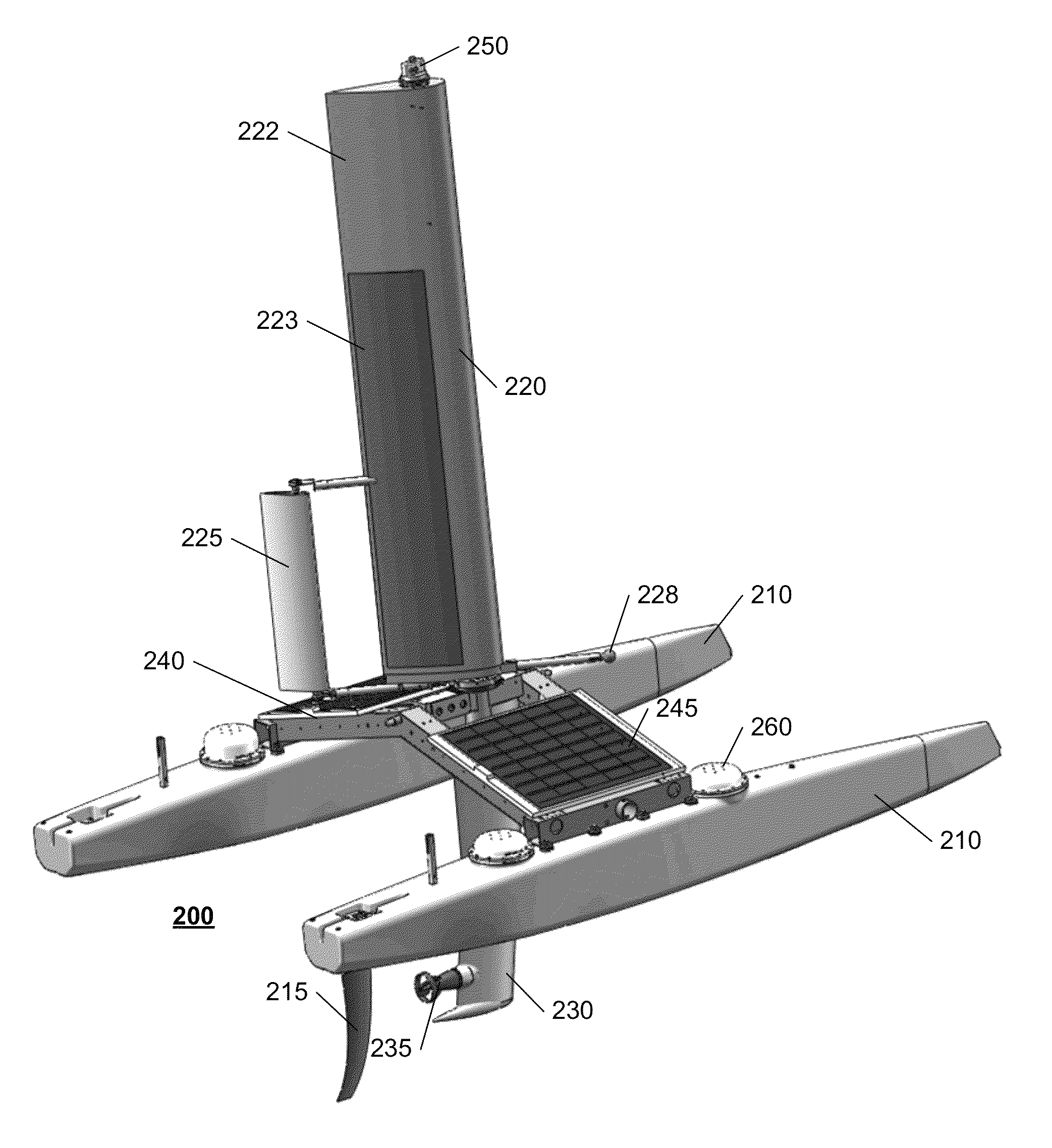
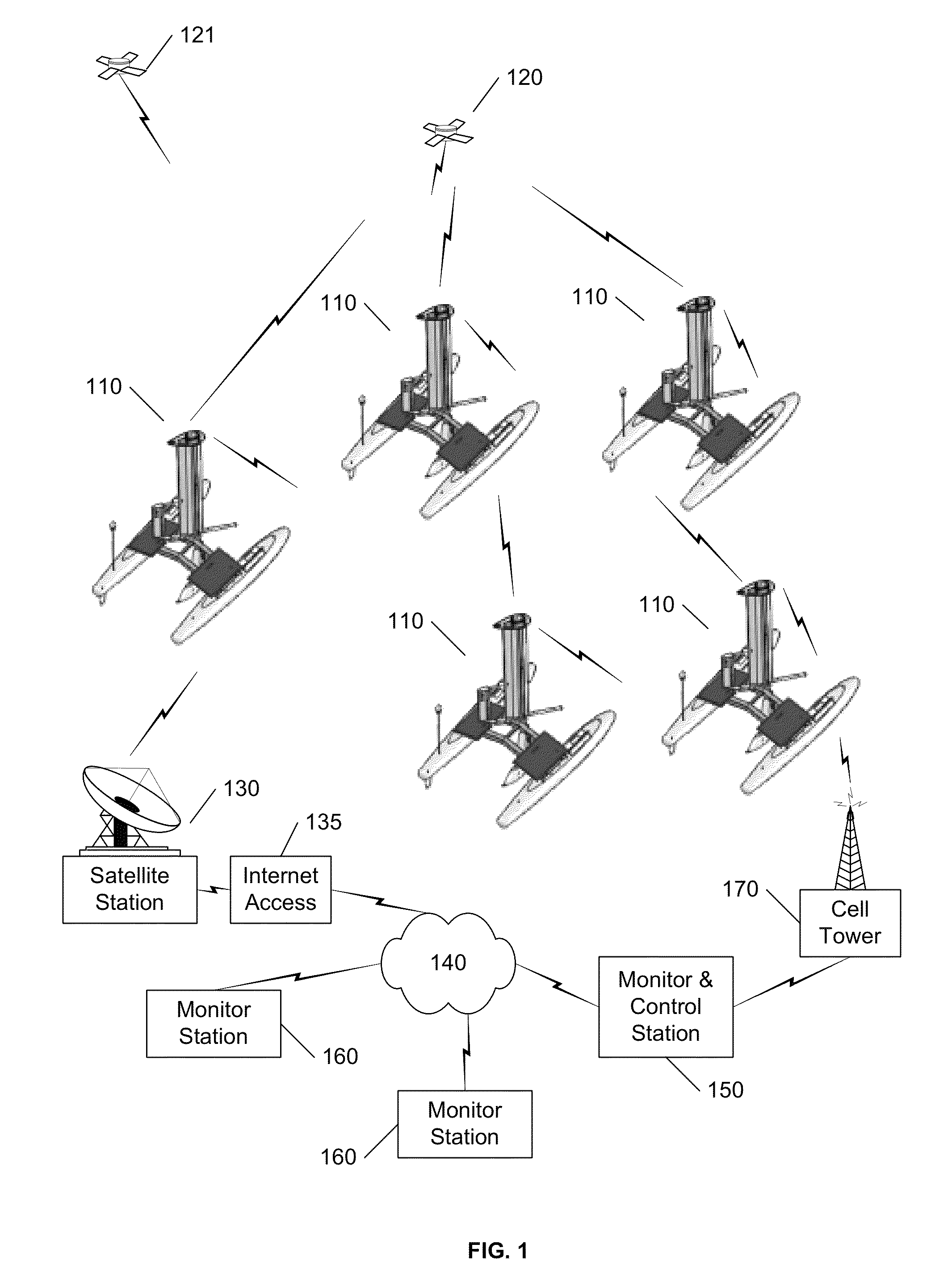
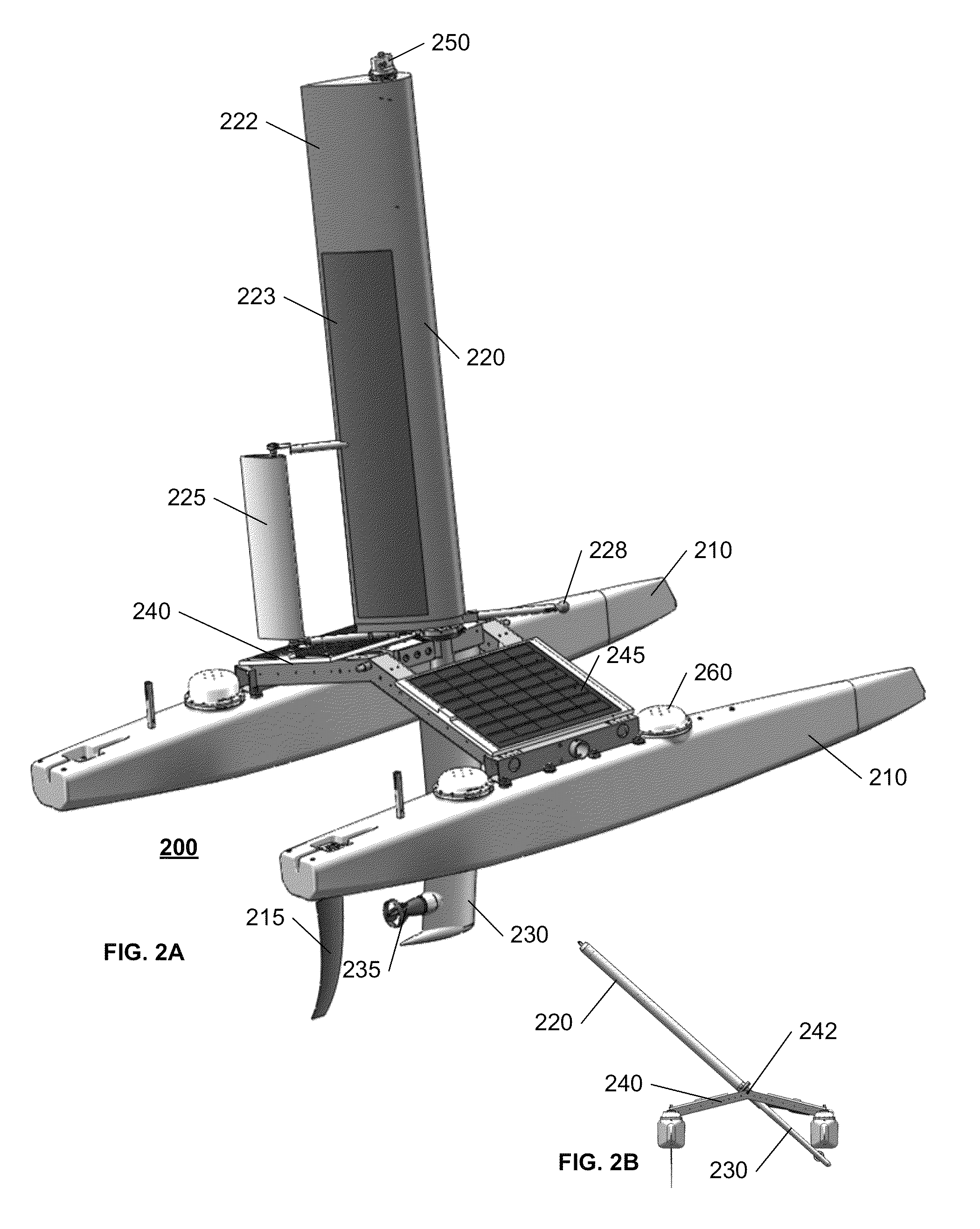
Autonomous sailboat for oceanographic monitoring
ActiveUS20140116311A1Efficient processAccurate monitoringAuxillariesWaterways transportMarine engineeringModularity
A fleet of autonomous sailing vessels that are equipped with monitoring and communication equipment for reporting environmental and other conditions. For optimal stability, the autonomous sailing vessels are multi-hulled vessels (catamarans) with self-righting capabilities. Each sailing vessel sends and receives information via one or more satellite links, using solar power to power the communications equipment as well as the monitoring equipment. Each sailing vessel includes an auto-sailtrim system to maintain a desired attack angle with the wind, and electric propulsion for use as required to maintain a desired heading. A modular design is used to support mission-specific payloads.
Owner:AUTONOMOUS MARINE SYST INC
Planting method of sweet potatoes
The invention relates to the technical field of crop planting, in particular to a planting method of sweet potatoes. The method comprises the steps of adding 25-40 kg of lime powder in per mu of cultivated land and conducting deep-ploughing treatment on the cultivated land, thickening soil layers to form pieces of ridges, planting two rows of sweet potato seedlings on each ridge in a mutually staggering mode, and scientifically fertilizing fertilizer. According to the planting method of the sweet potatoes, by adding the lime powder into the cultivated land, and using a rake to conduct the deep-ploughing treatment on the cultivated land, the lime powder is effectively and sufficiently mixed with soil, and thus the purpose of preventing insect damage is achieved; on each single ridge, two adjacent rows of sweet potatoes are planted in a staggering mode, thus it is guaranteed that nutrients in a field are sufficiently absorbed, and the situation that sweet potato seedlings which are planted side by side are mutually interfered and influenced is avoided. According to the planting method of the sweet potatoes, the sweet potatoes are high in yield, developed in root system, and high in fertilizer adsorbing capacity, the insect damage is effectively prevented, root promoting and seedling protection are achieved, the sweet potato seedlings are bright in leaf, the harvested sweet potatoes are excellent in overall quality, and high in yield, and thus the planting method of the sweet potatoes is suitable for agricultural popularization.
Owner:蒙运绍
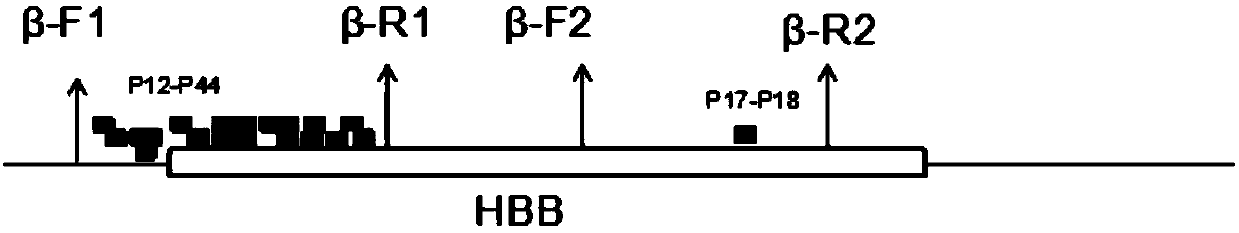
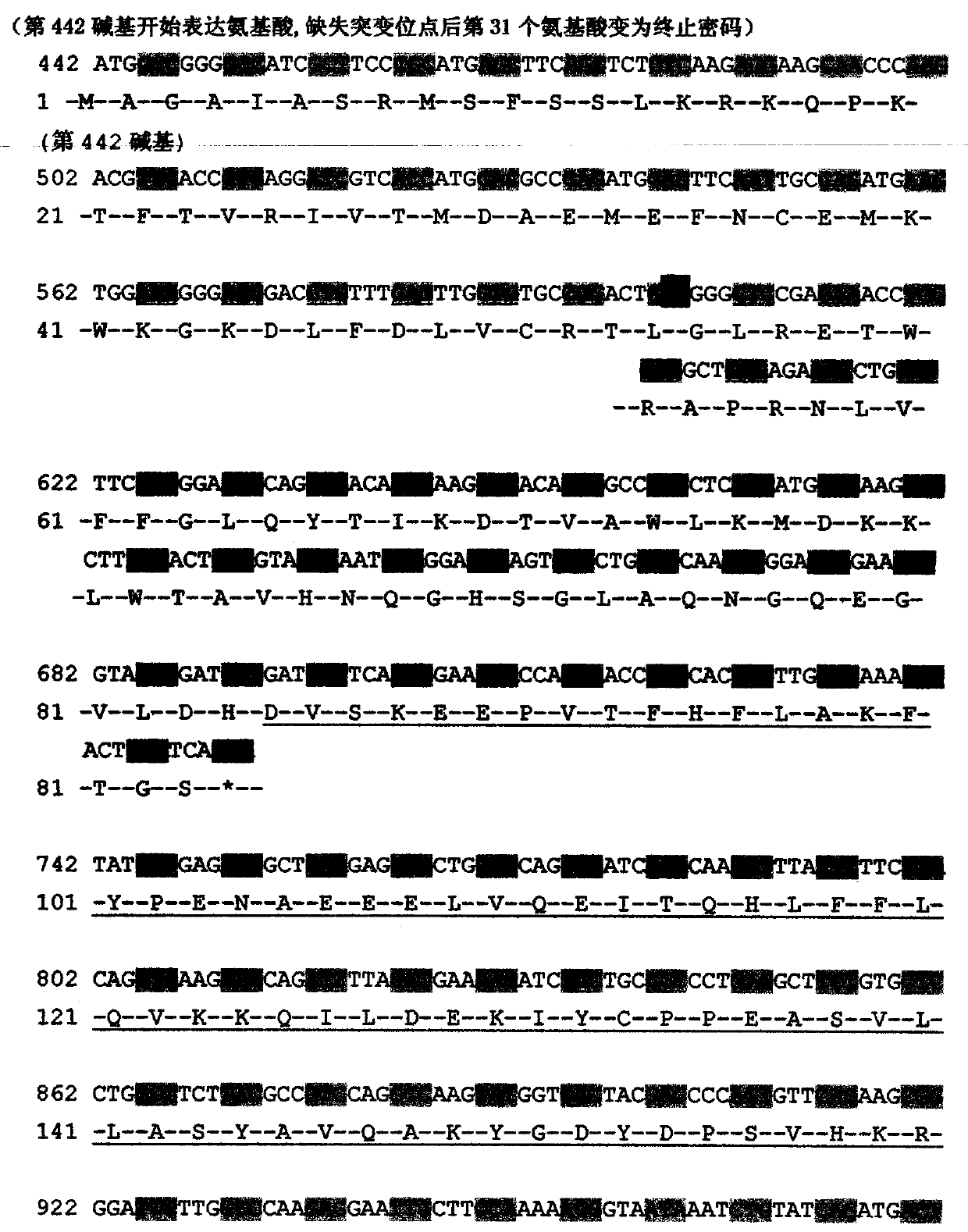
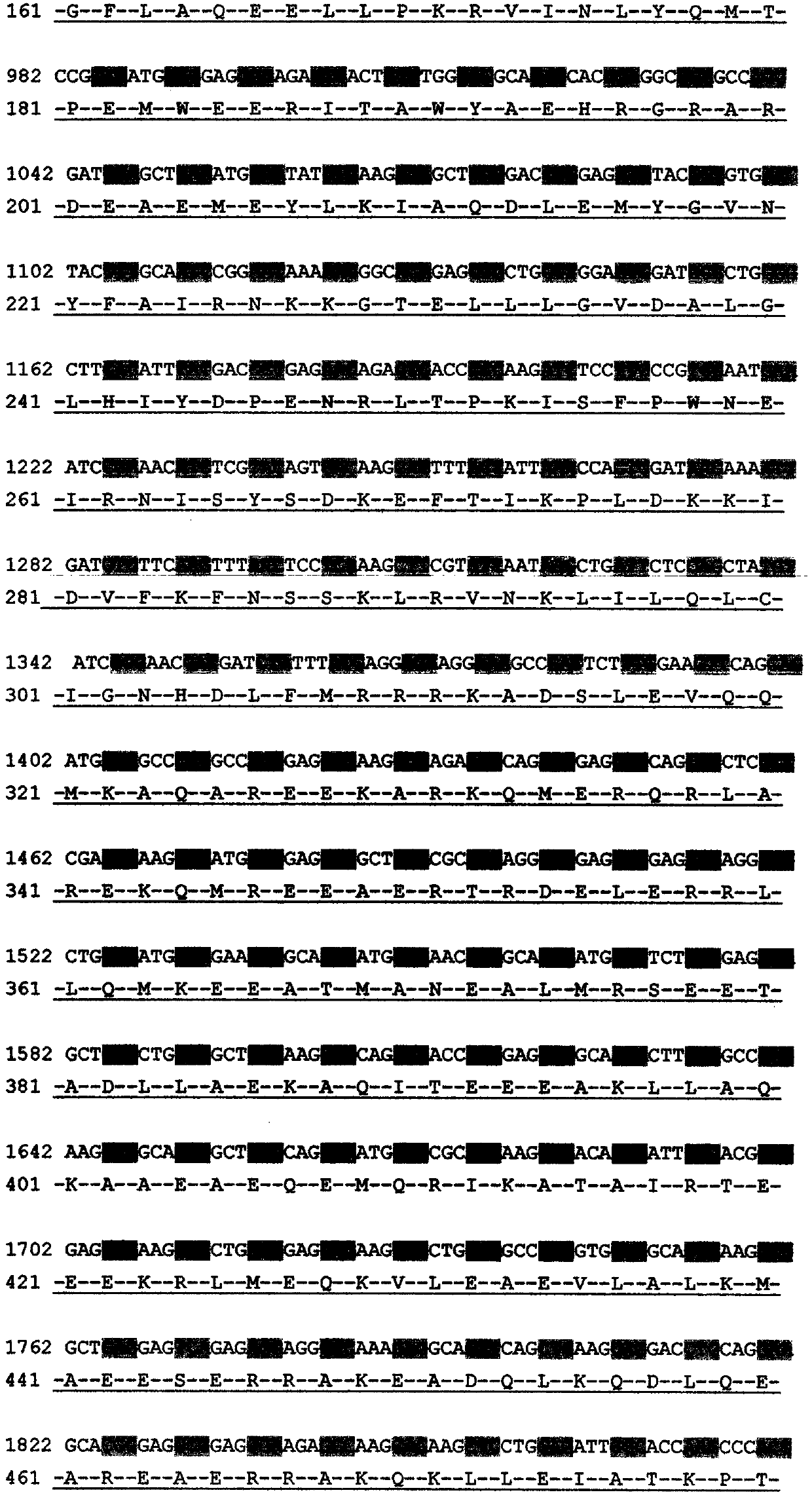
Mutation detection kit for sensorineural deafness virulence gene GJB2
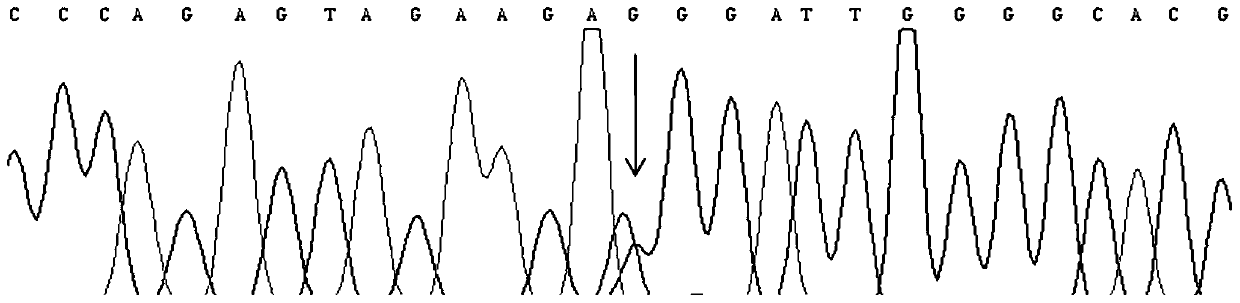
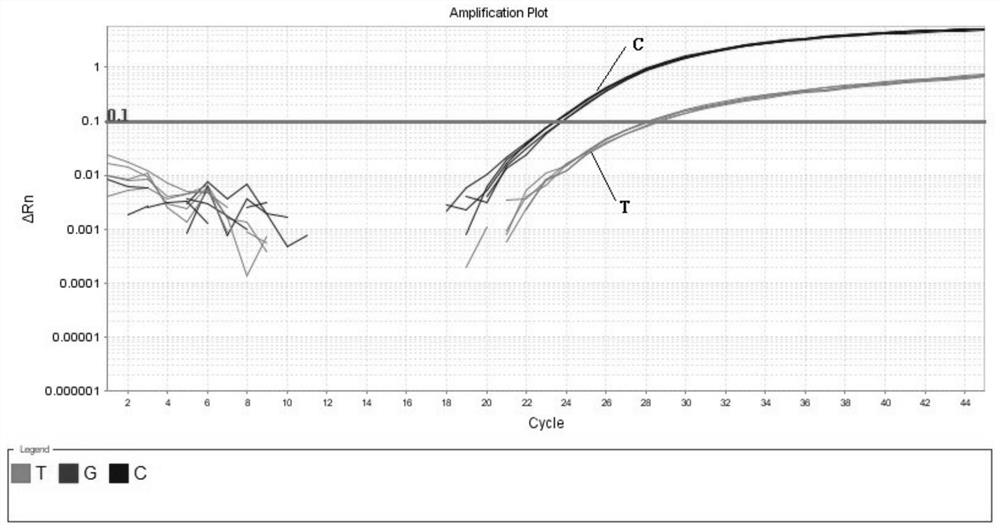
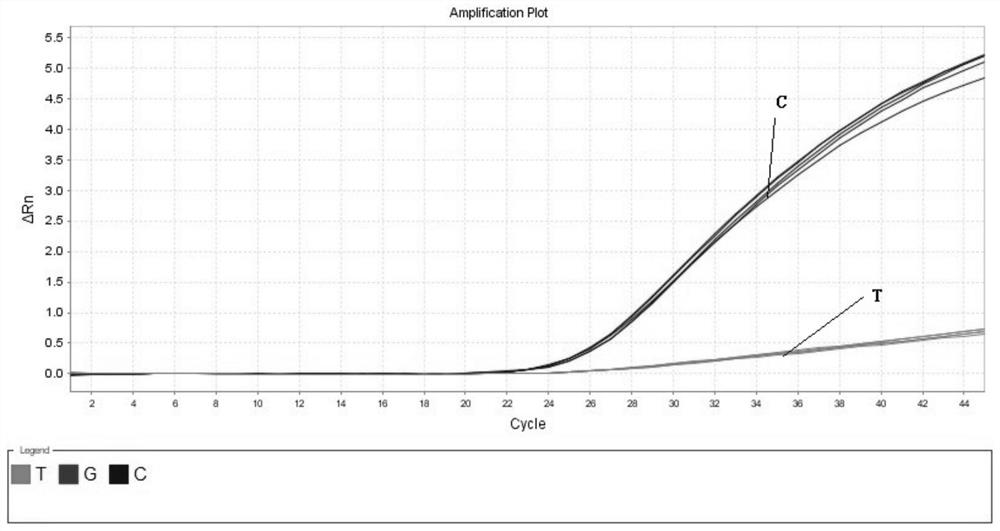
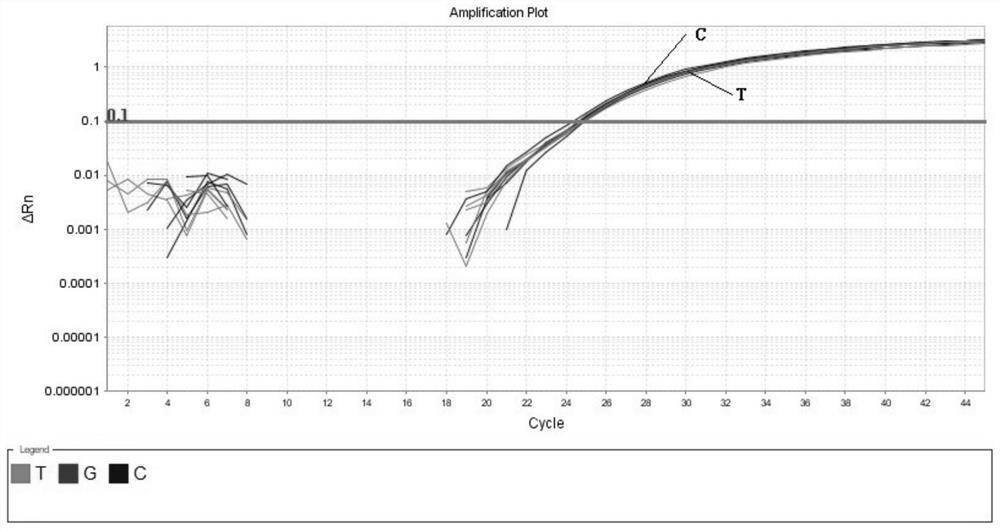
InactiveCN109251979ADiagnosis of sensorineural hearing lossReduce birth rateMicrobiological testing/measurementVirulent characteristicsMutation detection
The invention discloses a mutation detection kit for a sensorineural deafness virulence gene GJB2. The kit includes a reagent for extracting DNA from a sample to be tested, a PCR reaction reagent foramplifying the sample DNA, and a reagent for sequencing a PCR amplification product, wherein the PCR reaction reagent for amplifying the sample DNA includes a PCR primer. The kit of the present invention is used to detect whether a patient has GJB2 gene c.170A>C mutation, so that the cause of sensorineural deafness is diagnosed. The kit facilitates clinical screening of GJB2 mutation of patients with sensorineural deafness, and provides a basis for diagnosis of patients with sensorineural deafness.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Autonomous sailboat for oceanographic monitoring
ActiveUS8973511B2Efficient processAccurate monitoringAuxillariesUnmanned surface vesselsMarine engineeringModularity
A fleet of autonomous sailing vessels that are equipped with monitoring and communication equipment for reporting environmental and other conditions. For optimal stability, the autonomous sailing vessels are multi-hulled vessels (catamarans) with self-righting capabilities. Each sailing vessel sends and receives information via one or more satellite links, using solar power to power the communications equipment as well as the monitoring equipment. Each sailing vessel includes an auto-sailtrim system to maintain a desired attack angle with the wind, and electric propulsion for use as required to maintain a desired heading. A modular design is used to support mission-specific payloads.
Owner:AUTONOMOUS MARINE SYST INC
Sweet potato cultivation method
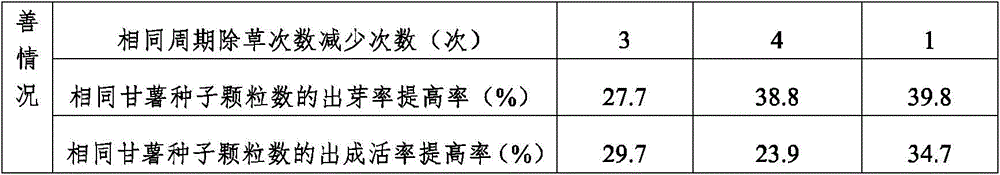
InactiveCN105940894AReduce the frequency of weedingGuaranteed growth qualityDi-calcium phosphate fertilisersAlkali orthophosphate fertiliserBiotechnologyNutrient solution
The invention relates to the technical field of potato cultivation, and specifically relates to a sweet potato cultivation method. Through the structural treatment upon a cultivation base during a sweet potato cultivation process, the sweet potato cultivation base can satisfy nutrition and nutrient supply needs during the sweet potato cultivation process, and weed overgrowth rate is also reduced, such that weeding frequency during the sweet potato cultivation process is reduced, high labor input is avoided, cultivation cost is reduced, and the damage to sweet potato seedlings due to excessive weeding treatments can be avoided. Therefore, sweet potato growth quality is ensured. With the application of substances such as a nutrient agent, plant ash, traditional Chinese medicine components, organic nutrients and an organic nutrient solution, and with the control over the positions of the substances in the structural layers of the base, the base can provide different nutrient components according to different sweet potato growth periods. Therefore, sweet potato growth can be rapidly improved, sweet potato yield can be improved, labor input can be reduced, and cost can be reduced.
Owner:贵州省印江县依仁食品有限公司
Human motor neuron gene copy number relative quantitative detection method and kit
PendingCN112280848AImprove accuracyGood repeatabilityMicrobiological testing/measurementDNA/RNA fragmentationMedicineSingle strand
The invention discloses a human motor neuron gene copy number relative quantitative detection method and a detection kit. The kit provided by the invention contains a single-stranded DNA group A for specifically detecting the seventh exon of the SMN gene and / or a single-stranded DNA group B for specifically detecting the eighth exon of the SMN gene, and each single-stranded DNA group consists of acommon primer and two probes for respectively detecting the SMN1 gene and the SMN2 gene. The kit is high in accuracy and good in repeatability. The method has important significance for rapidly screening SMN1 gene and SMN2 copy number and reducing birth rate of SMA children patients.
Owner:北京迈基诺基因科技股份有限公司
Nucleic acid composition and detection kit for detecting genetic anemia as well as use method
ActiveCN108796042AAffect the effect of amplificationImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationBeta thalassemiaMutation detection
The invention relates to a nucleic acid composition and a detection kit for detecting genetic anemia as well as a use method. The nucleic acid composition and the detection kit for detecting the genetic anemia can be used for simultaneously detecting four deletion type alpha thalassemia genes, three non-deletion type alpha thalassemia genes, nineteenth mutation type beta thalassemia genes, geneticanemia types such as sickle-shaped thalassemia gene as well as specific gene mutation types. Compared with the prior similar technologies, the detection kit for the genetic anemia has the characteristics that several mutation detection types for the genetic anemia are increased, such as alphaTHAI deleted thalassemia, sickle-shaped thalassemia and 71 / 72(+T) mutation and -28M(A-C) mutation which are relatively-rare thalassemia types. The detection for the genetic anemia types can provide visual reference and prompt for clinically detecting the genetic anemia, so that the leak detection risk ofclinical genetic anemia can be greatly reduced and the birth rate of severe anemia children is reduced.
Owner:BEIHAO STEM CELL & REGENERATIVE MEDICINE RES INST CO LTD
Phenylketonuria (PKU) screening kit and application thereof to prenatal screening
InactiveCN103276077AShorten screening timeScreening is quick and easyMicrobiological testing/measurementGeneticsPrenatal screening
The invention discloses a phenylketonuria (PKU) screening kit and application thereof to prenatal screening, and belongs to the technical field of PKU screening. The application method comprises the following steps of: (1) extracting free fetal DNA (Deoxyribonucleic Acid) from peripheral blood of pregnant women; (2) taking the fetal DNA as a template to carry out polymerase chain reaction (PCR) amplification; (3) detecting a PCR product through agarose gel electrophoresis and purifying the PCR product through enzyme reaction; (4) carrying out sequencing reaction on the purified PCR product; (5) purifying a PCR sequencing reaction product; (6) sequencing the purified PCR sequencing reaction product; and (7) comparing the sequencing result with positive and negative control samples. The kit has the beneficial effects that the kit can achieve automatic sequencing, thus shortening the screening time and enabling PKU screening to be simpler, more convenient and faster; the technical gap of prenatal PKU screening is filled; the birth rate of sick children is reduced; and the kit causes no trauma to the fetuses and is safe and reliable.
Owner:邯郸市康业生物科技有限公司
Kit for external detection of Neurofibromastosis 2 disease causative gene NF2 c.1598delA mutation
InactiveCN104878079AReduce birth rateEasy to diagnoseMicrobiological testing/measurementDNA/RNA fragmentationPrenatal screeningBiology
The invention discloses a kit for external detection of Neurofibromastosis 2 disease causative gene NF2 c.1598delA mutation. The kit comprises a primer pair shown in the formulas of SEQ ID NO: 1 and SEQ ID NO: 2. The invention firstly discloses Neurofibromastosis 2 disease causative gene NF2 c.1598delA mutation and proves a relationship of the mutation site and the Neurofibromastosis 2 disease. The invention further designs the primers for detecting the mutation site and discloses a use of the primers in diagnosis of the Neurofibromastosis 2 disease. Through prenatal screening and neonatal NF2 gene mutation screening, a birth rate of the children suffering from the NF2 disease is reduced, disease is prevented and social pressure is greatly reduced.
Owner:BEIJING TIANTAN HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
Kit for detecting mutation of pathogenic gene GJB2 of sensorineural deafness
InactiveCN109554463ADiagnosis of sensorineural hearing lossReduce birth rateMicrobiological testing/measurementSensorineural deafnessDNA
The invention discloses a kit for detecting mutation of a pathogenic gene GJB2 of sensorineural deafness. The kit includes reagents for extracting DNA from a sample to be tested, PCR reaction reagentsfor amplifying the sample DNA, and reagents for sequencing PCR amplified products. The PCR reaction reagents for amplifying the sample DNA include PCR primers. The kit can be used for detecting the mutation of the GJB2 gene c.67dupA in patients and diagnosing the cause of sensorineural deafness. The kit can be helpful for carrying out screening work of the mutation of GJB2 in patients with sensorineural deafness in clinic and provides a basis for the diagnosis of the patients with sensorineural deafness.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Manipulator for cloth feeding
ActiveCN108724803AAvoid size differencesReduce the probability of occurrencePress ramEngineeringMechanical equipment
The invention discloses a manipulator for cloth feeding, and relates to the field of mechanical equipment. The manipulator is simple in structure, low in cost and convenient to operate. The manipulator comprises a material grasping mechanism, a material sending mechanism, a material placement platform and a supporting frame. The material grasping mechanism and the material sending mechanism are separately arranged at the two ends of the material placement platform. The material grasping mechanism comprises a telescopic adjusting assembly, a horizontal moving assembly and a vertical lifting assembly. The horizontal moving assembly is connected with the supporting frame; the vertical lifting assembly is connected with the horizontal moving assembly; the telescopic adjusting assembly is connected with the vertical lifting assembly; and the telescopic adjusting assembly is connected with a material grasp piece. The material sending mechanism comprises a horizontal adjusting assembly, a lifting adjusting assembly, a moving material sending assembly and material sending beams. A material grasp piece of the material grasping mechanism grasps to-be-conveyed cloth materials, the material grasping mechanism drives the to-be-conveyed cloth materials to move and places the to-be-conveyed cloth materials onto the material placement platform, and the material sending beams of the material sending mechanism clamp the to-be-conveyed cloth materials and move to a target location.
Owner:山东博锐机器人科技有限公司
Kit for detecting mutation of pathogenic gene POU3F4 of inner-ear malformation/incomplete separation type-III cochlear malformation
ActiveCN109652536AReduce birth rateReduce the burden onMicrobiological testing/measurementDNA/RNA fragmentationCochlear malformationMutation screening
The invention discloses a kit for detecting the mutation of a pathogenic gene POU3F4 of inner-ear malformation / incomplete separation type-III cochlear malformation. The kit comprises a reagent for extracting DNA from a sample to be detected, a PCR reaction reagent for amplifying the DNA of the sample, and a reagent for sequencing a PCR amplification product. The PCR reaction reagent for amplifyingthe DNA of the sample comprises a PCR primer. The kit is used for detecting whether or not the mutation of the POU3F4 gene c.456 -469 del14 happens to a patient so as to diagnose the cause of the occurrence of inner-ear malformation / incomplete separation type-III cochlear malformation. The kit is beneficial to clinical implementation of POU3F4 mutation screening work on patients suffering from inner-ear malformation / incomplete separation type-III cochlear malformation and provides a basis for the diagnosis of the patients suffering from inner-ear malformation / incomplete separation type-III cochlear malformation.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Detection kit for pathogenic gene CHD7 mutation of CHARGE syndrome
PendingCN110029161AReduce birth rateReduce the burden onMicrobiological testing/measurementCHARGE syndromeMutation screening
The invention discloses a detection kit for a pathogenic gene CHD7 mutation of the CHARGE syndrome. The kit comprises a reagent for extracting DNA from a to-be-detected sample, a PCR reaction reagentfor amplifying the sample DNA, and a reagent for sequencing a PCR amplified product, wherein the PCR reaction reagent for amplifying the sample DNA comprises a PCR primer. The kit is used for detecting whether a patient has mutation of NM_017780:c.3523-2A>G of a CHD7 gene or not, and the cause of CHARGE syndrome occurrence can be diagnosed. The kit is favorable to clinically performing CHD7 mutation screening operation of patients with CHARGE syndrome, and accordance is provided to diagnosis for patients with the CHARGE syndrome.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Kit for LVA (Large Vestibular Agueduct)/Pendred syndrome virulence gene SLC26a4 mutation detection
PendingCN109680057AReduce birth rateReduce the burden onMicrobiological testing/measurementPipingPolymerase chain reaction
The invention discloses a kit for LVA (Large Vestibular Agueduct) / Pendred syndrome virulence gene SLC26a4 mutation detection. The kit is prepared from a reagent which is used for extracting DNA (Deoxyribonucleic Acid) from a to-be-detected sample, a PCR (Polymerase Chain Reaction) reagent which is used for amplifying the DNA of a sample and a reagent for sequencing a PCR amplification product, wherein the PCR reagent for amplifying the DNA of the sample comprises a PCR primer. The kit disclosed by the invention is used for detecting whether SLC26A4 gene c.85G>A exists in a patient or not, so that the reason causing the LVA / Pendred syndrome can be diagnosed; the kit will be beneficial for clinically developing SLV26A4 mutation detection work on the patient suffering from the LVA / Pendred syndrome, and basis is provided for diagnosis on the patient suffering from the LVA / Pendred syndrome.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Pregnancy full-nutrition eugenics paste
InactiveCN105661526AImprove nutritional statusReduce complicationsFood ingredient functionsDigestionBiology
The invention relates to a health-care food, and in particular discloses pregnancy full-nutrition eugenics paste. The pregnancy full-nutrition eugenics paste is prepared from the following raw materials in certain parts by weight: gorgon euryale seed, red peanut, soybean, red kidney bean, chickpea, Chinese chestnut, lotus seed, longan pulp, black sesame, date, walnut kernel, yellow rice, red rice, millet, sorghum rice, selenium-rich rice, and black glutinous rice. The pregnancy full-nutrition eugenics paste has the advantages that after eating for a long time, the nutrition level of a pregnant woman is favorably improved, the normal development of a fetus is guaranteed, the complication of the pregnant woman is reduced, the condition of low-weight children and the birth rate of deformed children are decreased, and the quality of population is favorably improved; the preparation is simple and convenient, the mouth feel is fragrant and sweet, the digestion and absorption are easy, and the pregnancy full-nutrition eugenics paste is suitable for the growth and development of pregnancy fetuses; after the pregnant woman eats the pregnancy full-nutrition eugenics paste, the body immunity is improved, the sleeping quality is improved, the appetite is increased, and a foundation is laid for the prenatal and postnatal care.
Owner:SHANDONG DAOZHIZIRAN HEALTH IND CO LTD
Protein related to human hair and tooth development amd its coding gene
InactiveCN1763088BReduce birth rateImprove population qualityBacteriaFermentationDiseasePrenatal diagnosis
The present invention discloses one kind of protein related to growth of human air and teeth and its coding gene. The protein related to growth of human air and teeth is protein of EDA with place 65 amino acid from the amino end mutated from arginine into glycine. The protein related to growth of human air and teeth and its coding gene of the present invention provide essentiality and possibilityfor the genetic diagnosis of X-linked congenital tooth deficiency disease, such as in antemarital consultation, DNA based antenatal diagnosis, etc. and this can reach the aim of raising population quality.
Owner:东营协和基因技术有限公司 +2
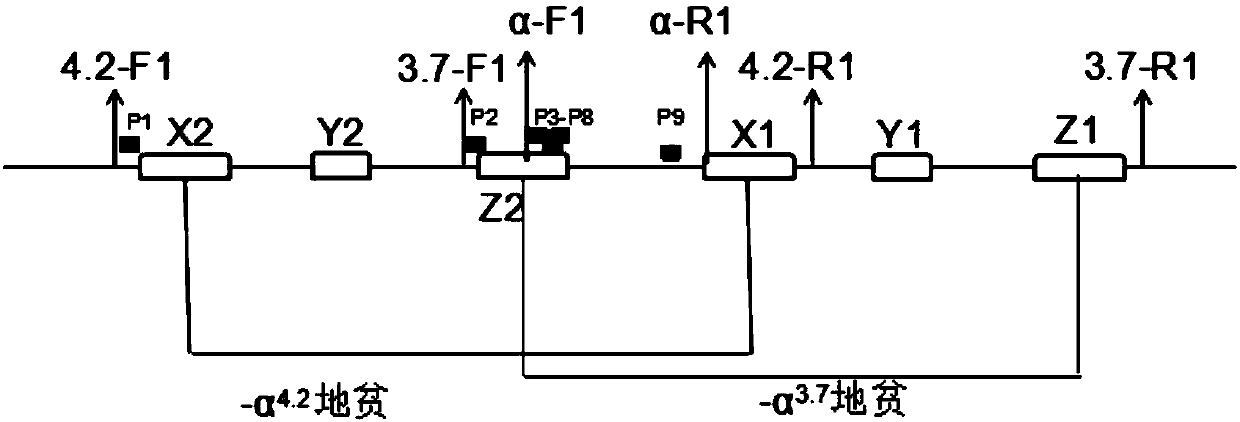
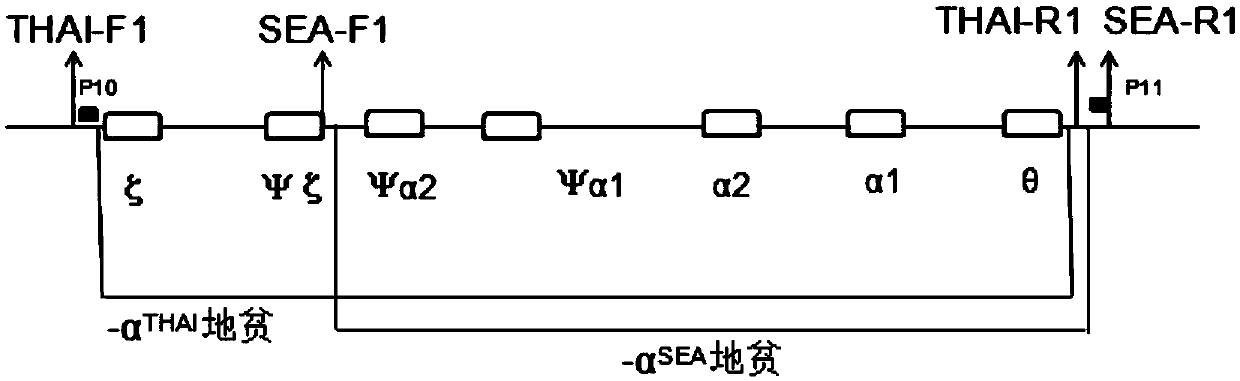
Alpha-thalassemia screening kit and application thereof in prenatal screening
ActiveCN103421903BReasonable primer designAchieving Prenatal ScreeningMicrobiological testing/measurementPrenatal screeningBiology
The invention discloses an α-thalassemia screening kit and its application in prenatal screening, belonging to the technical field of α-thalassemia prenatal screening. The α-thalassemia screening kit includes: (1) Negative control sample; (2) Positive control sample; (3) PCRMasterMIX; (4) PCR primers. Its application uses designed primers to amplify fetal DNA in the peripheral blood of pregnant women to achieve prenatal screening for α-thalassemia. The present invention collects the peripheral blood of pregnant women for prenatal screening of α-thalassemia, without puncturing the amniotic cavity and inserting villi tissue, without any trauma to the fetus, and is safe and reliable; the accuracy rate can reach 99.99%, filling the non-invasiveness of α-thalassemia The technical gap in prenatal screening reduces the birth rate of sick children.
Owner:邯郸市康业生物科技有限公司
Kit for detecting folic acid related gene polymorphism and using method thereof
PendingCN110684832AStrong specificityReduce pollutionMicrobiological testing/measurementDNA/RNA fragmentationWild typePolymorphism Detection
The invention relates to the field of gene polymorphism detection, in particular to a kit for detecting folic acid related gene polymorphism and a using method thereof. The kit includes a nucleic acidamplification reagent, and the nucleic acid amplification reagent includes a folic acid wild-type reaction solution, a folic acid mutant-type reaction solution and a mixed enzyme solution. The folicacid wild-type reaction solution contains primers and probes for detecting wild-type genes, and the folic acid mutant-type reaction solution contains primers and probes for detecting mutant-type genes. The kit has the technical advantages of high sensitivity, high specificity and good stability.
Owner:苏州云泰生物医药科技有限公司 +1
Kit for detecting mutation of sensorineural deafness pathogenic gene GJB2
ActiveCN109628574ADiagnosis of sensorineural hearing lossReduce birth rateMicrobiological testing/measurementDNA/RNA fragmentationMutation screeningSensorineural deafness
The invention discloses a kit for detecting mutation of a sensorineural deafness pathogenic gene GJB2. The kit comprises a reagent for extracting DNA from a sample to be detected, a PCR reaction reagent for amplifying the DNA of the sample, and a reagent for sequencing a PCR amplification product; wherein the PCR reaction reagent for amplifying the DNA of the sample comprises a PCR primer. The kitis used for detecting whether a GJB2 gene c. 142C) T mutation exists in a patient so as to diagnose the cause of sensorineural deafness; the kit facilitates clinically developing GJB2 mutation screening work of sensorineural deafness patients, and provides a basis for the diagnosis of sensorineural deafness.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Nucleic acid composition, detection kit and method of use for detection of hereditary anemia
ActiveCN108796042BReduce the risk of missed detectionReduce birth rateMicrobiological testing/measurementDNA/RNA fragmentationPhysiologyMutation detection
Owner:BEIHAO STEM CELL & REGENERATIVE MEDICINE RES INST CO LTD
Primer group, probe group and kit for detecting high-frequency gene pathogenic variation
ActiveCN113265461ADiagnose as soon as possibleReduce birth rateMicrobiological testing/measurementDNA/RNA fragmentationFertility riskFertility
The invention belongs to the technical field of biological detection, and discloses a primer group, a probe group and a kit for detecting high-frequency gene pathogenic variation. The kit can conveniently and rapidly assist a doctor in providing scientific data or providing data support for carrier screening when the doctor diagnoses a patient, and is helpful for the doctor to diagnose the patient as soon as possible or predict fertility risks in advance in a pregnancy preparation stage. The kit is a means for detecting the genetic material DNA / RNA, is high in accuracy, low in false positive rate, low in cost and simple in result interpretation, and effectively reduces the birth rate of children suffering from the kraber disease. The amplification efficiency difference between alleles of the reaction system is maximized, and the reaction system is suitable for Ct value interpretation and is also suitable for end-point method interpretation; besides, the consumed time is short and the accuracy is high. The kit can be used for rapidly and accurately detecting c.1901T > C pathogenic variation on a GALC gene NM_000153 transcript in a human whole blood sample, and is used for auxiliary diagnosis of the Kraber disease.
Owner:北京华诺奥美医学检验实验室有限公司
A primer set, probe set and kit for detecting high-frequency gene pathogenic variants
ActiveCN113265461BDiagnose as soon as possibleReduce birth rateMicrobiological testing/measurementDNA/RNA fragmentationPregnancyWhole blood sample
The invention belongs to the technical field of biological detection, and discloses a primer set, a probe set and a kit for detecting high-frequency gene pathogenic variation. The kit can conveniently and quickly assist doctors to provide scientific data when diagnosing patients or provide data support for carrier screening, which helps doctors diagnose patients as soon as possible, or predict fertility risks in advance during the pregnancy preparation stage. It is a method for detecting genetic material DNA / RNA, which has high accuracy, low false positive rate, low cost, simple interpretation of results, and effectively reduces the birth rate of children with Krabbe disease. The reaction system maximizes the difference in amplification efficiency between alleles, and is suitable for Ct value interpretation and endpoint method interpretation; it takes less time and has high accuracy. It can be used to quickly and accurately detect the c.1901T>C pathogenic variant on the GALC gene NM_000153 transcript in human whole blood samples, and is used for the auxiliary diagnosis of Krabbe disease.
Owner:北京华诺奥美医学检验实验室有限公司
Gene mutation combination serving as marker of MRKH syndrome and application of gene mutation combination
ActiveCN112695082AAvoid birthReduce incidenceMicrobiological testing/measurementImmunoglobulins against animals/humansPhysiologyMedical diagnosis
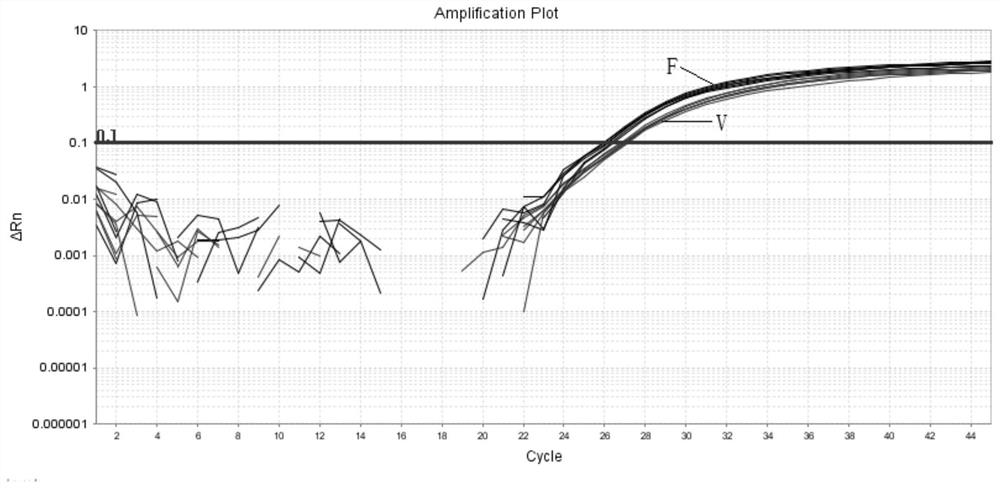
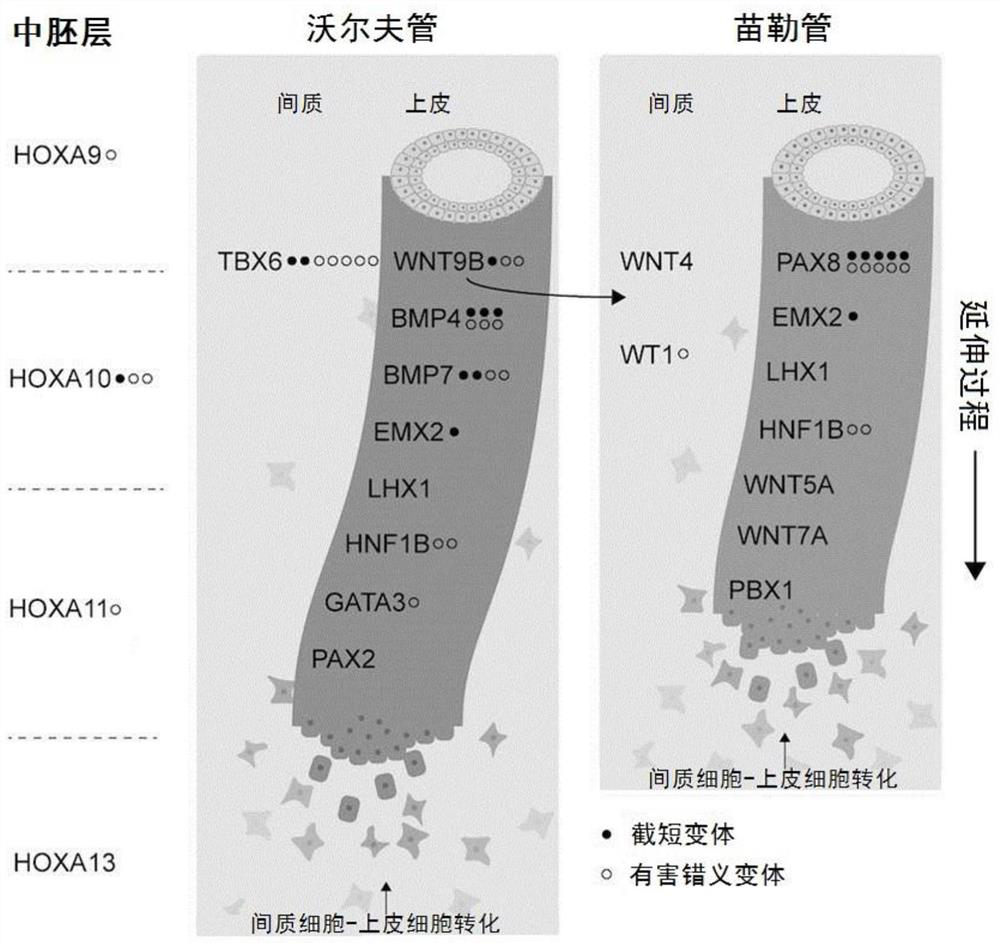
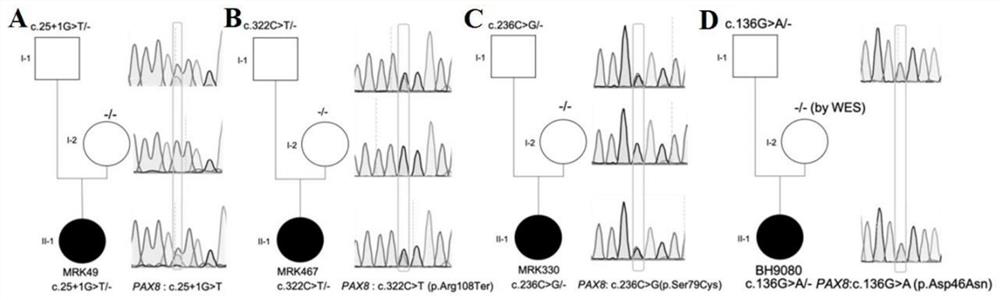
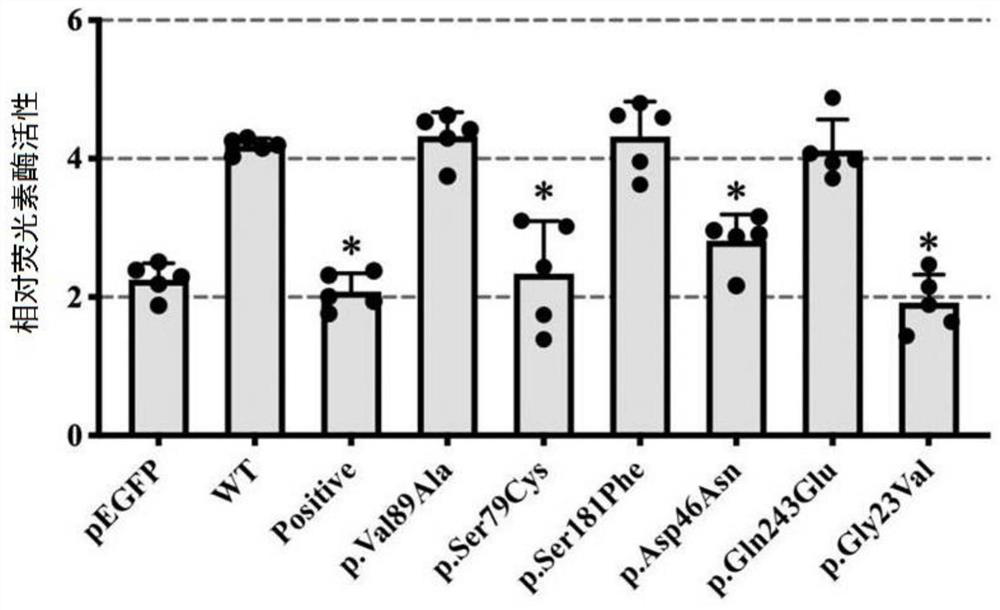
The invention discloses a gene mutation combination serving as a marker of the MRKH syndrome and application of the gene mutation combination, and relates to the field of biotechnology and medical diagnosis. The gene mutation combination is a group of pathogenic gene mutation sites related to the MRKH syndrome. According to the research of the invention, by whole exome sequencing, the following mutation sites on a gene PAX8 of a patient with the rare MRKH syndrome are found in the world for the first time: c.236C> G, c.156_157dupCG, c.25+1G> T, c.195delC, c.322C> T and c.542C> T; and experimental verification shows that the mutant gene PAX8 carrying the mutation sites can be used as the diagnosis marker of the MRKH syndrome, is used for diagnosing whether the patient suffers from the MRKH syndrome or not and carrying out pre-pregnancy early warning, and provides a brand-new thought for researching the pathogenesis of the MRKH syndrome.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Markers for labour
InactiveUS20080233106A1Suppress uterine contractionReduce laborBioreactor/fermenter combinationsBiological substance pretreatmentsAssayPathology
The invention relates to markers which find use in the diagnosis of labour or pre-term labour, to assays comprising such markers, to methods of identifying therapeutic agents which can prolong pregnancy, using these markers and to methods of treatment of pre-term labour, methods of prolonging gestation, or methods of suppressing labour contractility based on the markers.
Owner:NATIONAL UNIVERSITY OF IRELAND
Marker Sequences for Labour
InactiveUS20100119504A1Suppress uterine contractionReduce laborOrganic active ingredientsPeptide librariesBiologyProlonged gestation
The invention relates to markers which find use in the diagnosis of labour or pre-term labour, to assays comprising such markers, to methods of identifying therapeutic agents which can prolong pregnancy, using these markers and to methods of treatment of pre-term labour, methods of prolonging gestation, or methods of suppressing labour contractility based on the markers.
Owner:NATIONAL UNIVERSITY OF IRELAND
Pharmaceutical composition capable of preventing and treating rodent pests and preparation method of pharmaceutical composition
InactiveCN106818899AReduce birth rateSolve the major problem of eating less and not giving birthBiocideAnimal repellantsDried fishMedicine
The invention discloses a pharmaceutical composition capable of preventing and treating rodent pests and a preparation method of the pharmaceutical composition. The pharmaceutical composition is columnar granules prepared from the following raw materials in percentage by weight: 0.2 percent to 0.9 percent of gossypol, 0.03 percent to 0.3 percent of ricinus communis agglutinin, 99 percent to 99.7 percent of a bait material, wherein the bait material is prepared from the following raw materials in percentage by weight: 15 percent to 30 percent of elm leaf and peel powder or pagoda tree leaf and flower powder, 35 percent to 55 percent of corncob powder or bran powder, 10 percent to 30 percent of carrot powder or peanut cake powder and 10 percent to 20 percent of chicken giblet powder or dried fish powder. According to the pharmaceutical composition capable of preventing and treating the rodent pests and the preparation method of the pharmaceutical composition, provided by the invention, a new mechanism of blood coagulation rodenticide is created and the effect that female and male rats are sterile after one-time of orally taking the medicine is enhanced; secondary poisoning is not caused and the environment pollution is reduced; the pharmaceutical composition has the effect of preventing and treating the rodent pests for a long period.
Owner:李健
Kit for detecting mutation of pathogenic gene KCNQ4 of non-syndromic autosomal dominant hereditary deafness
InactiveCN109554464AReduce birth rateAvoid economic lossMicrobiological testing/measurementDNAHereditary deafness
The invention discloses a kit for detecting mutation of a pathogenic gene KCNQ4 of non-syndromic autosomal dominant hereditary deafness. The kit includes a reagent for extracting DNA from a sample tobe tested, a PCR reaction reagent for amplifying the sample DNA, and a reagent for sequencing PCR amplification products. The PCR reaction reagent for amplifying the sample DNA includes a PCR primer.The kit is used for detecting the existence of c.C1258T mutation of the KCNQ4 gene of a patient, so the reason of non-syndromic autosomal dominant hereditary deafness is diagnosed. The kit can be helpful for carrying out screening work of KCNQ4 mutation in the patient with non-syndromic autosomal dominant deafness in clinic, and provides a basis for diagnosis of the patient with non-syndromic autosomal dominant hereditary deafness.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
A kit for in vitro detection of the c.602deltg mutation of the neurofibromastosis 2 disease-causing gene NF2
InactiveCN104878080BEasy to diagnoseLow costMicrobiological testing/measurementDiseasePrenatal screening
The invention discloses a kit for external detection of Neurofibromastosis 2 disease causative gene NF2 c.602delTG mutation. The kit comprises a primer pair shown in the formulas of SEQ ID NO: 1 and SEQ ID NO: 2. The invention firstly discloses Neurofibromastosis 2 disease causative gene NF2 c.602delTG mutation and proves a relationship of the mutation site and the NF2 disease. The invention further designs the primers for detecting the mutation site and discloses a use of the primers in diagnosis of the NF2 disease. Through prenatal screening and neonatal NF2 gene mutation screening, a birth rate of the children suffering from the NF2 disease is reduced, behavior of the patients with the causative gene is guide, disease is prevented and social pressure is greatly reduced. The kit for external detection of Neurofibromastosis 2 disease causative gene NF2 c.602delTG mutation is necessary for disciplinary research and development.
Owner:BEIJING TIANTAN HOSPITAL AFFILIATED TO CAPITAL MEDICAL UNIV
Photosensitive nerve deafness virulence gene GJB2 mutation detection kit
PendingCN109825577ADiagnosis of sensorineural hearing lossDetermine the cause of sensorineural hearing lossMicrobiological testing/measurementVirulent characteristicsMutation detection
The invention provides a photosensitive nerve deafness virulence gene GJB2 mutation detection kit. The kit comprises a reagent for extracting DNA from a sample to be detected, a PCR reaction reagent for amplifying a sample DNA, and a reagent for performing sequencing on a PCR amplification product; the PCR reaction reagent for amplifying the sample DNA comprises a PCR primer. The kit is used for detecting whether a patient has GJB2 gene c.2T>G mutation or not, and therefore the reason for the photosensitive nerve deafness is diagnosed. The kit facilitates GJB2 mutation screening work for the patient with the photosensitive nerve deafness, and the basis is provided for diagnosing the patient with the photosensitive nerve deafness.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Primer and probe composition and kit for detecting achondroplasia related FGFR3 gene variation sites
PendingCN113981075AReduce birth rateImprove accuracyMicrobiological testing/measurementDNA/RNA fragmentationFgfr3 geneNucleotide
The invention discloses a primer and probe composition and a kit for detecting achondroplasia related FGFR3 gene variation sites. The related FGFR3 gene variation sites comprise a first site and a second site, wherein the first site is NM_000142.5: exon10: c.1138G > A: p.Gly380Arg, and the second site is NM_000142.5: exon10: c.1138G > C: p.Gly380Arg; the nucleotide sequence of an upstream primer of the first site and the second site is as shown in SEQ ID NO.1, and the nucleotide sequence of a downstream primer of the first site and the second site is as shown in SEQ ID NO.2; the nucleotide sequence of a wild probe at the first site and a wild probe at the second site is shown as SEQ ID NO.3, and the nucleotide sequence of a mutation probe at the first site is shown as SEQ ID NO.4; and the nucleotide sequence of a mutation probe at the second site is as shown in SEQ ID NO.5. The primer and probe composition is high in accuracy, low in false positive rate, low in cost, free of high-end instruments, simple in result interpretation, suitable for general screening and capable of reducing the birth rate of children suffering from achondroplasia.
Owner:BEIJING JISHUITAN HOSPITAL +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com