Detection kit for pathogenic gene CHD7 mutation of CHARGE syndrome
A kit and genetic technology, applied in the field of genetic testing, to reduce the birth rate and reduce the burden
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
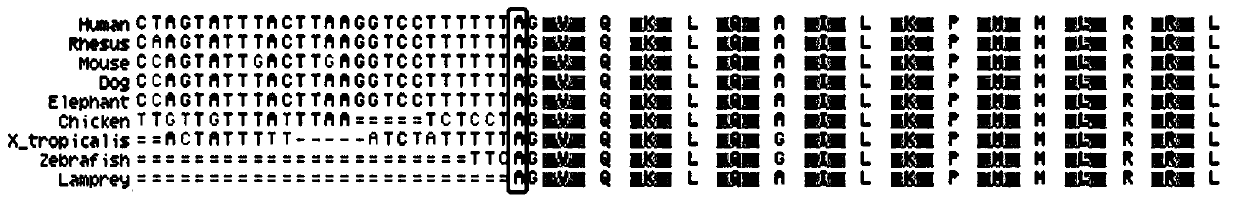
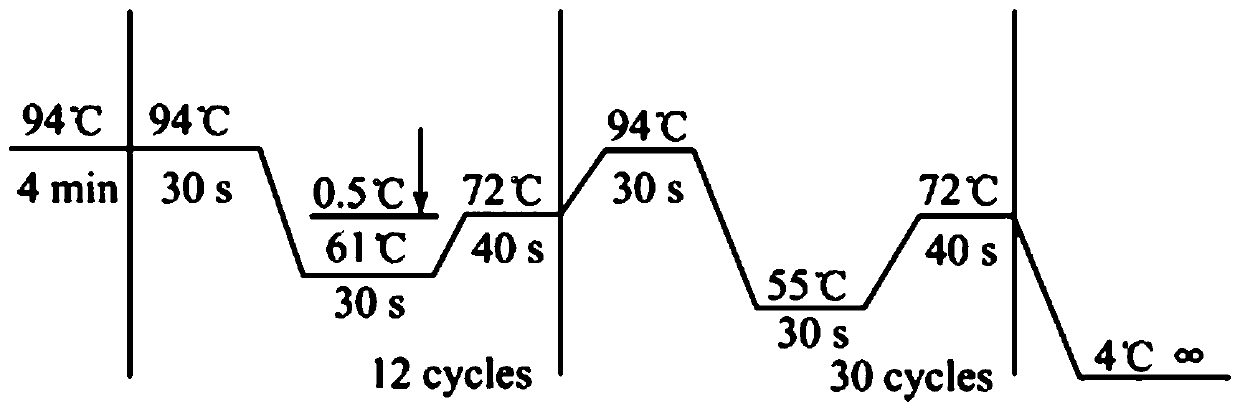
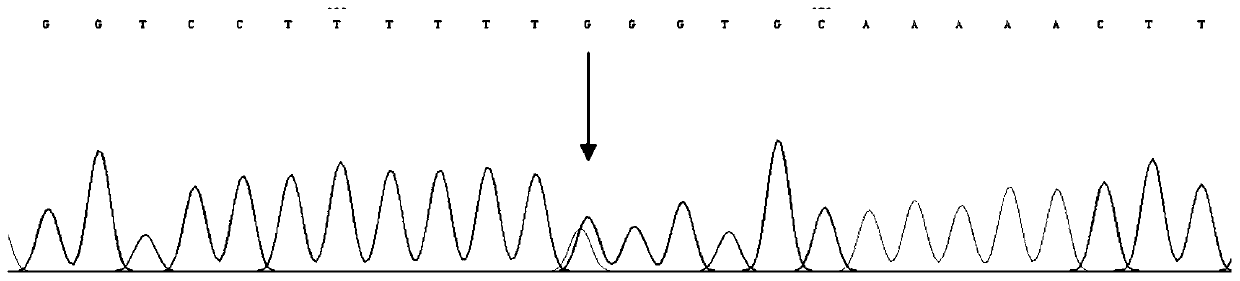
[0043] Collect all kinds of sensorineural deafness patients through the deaf clinic and resource collection network, and establish a resource library. On the premise that the patient is voluntary, after signing the informed consent, blood samples are collected, and an outpatient medical record database is established to record the patient's condition, family history and contact information in detail. Then, the genomic DNA was extracted by protease degradation, quantified and stored at -20°C. Each DNA sample corresponds to the registered patient's clinical data in detail. Then, use the online primer design software Primer5.0 to design primers (primer amplified target sequence contains exon 15 cut position of human CHD7 gene, primer design reference Gene ID: 55636, amplified target fragment size is 702bp), with genome DNA was used as a template, and PCR amplification was performed on a BIORAD MyCycle thermal cycler. Direct sequencing of PCR amplification products: the sequencin...
example 2
[0149] Amplification primers (design completed in September 2018) are as follows, and others are the same as Example 1 (the target sequence amplified by the following primers includes the cut position of exon 15 of the human CHD7 gene, and the primer design refers to Gene ID: 55636):
[0150] Upstream primer CHD7-F-2: 5'-CCCTCTTCTCTATCTTCCCTATG-3',
[0151] Downstream primer CHD7-R-2: 5'-GGACAGGAGACAGGAATGACTATA-3'.
[0152] The Fourth Military Medical University of the Chinese People's Liberation Army
[0153] CHARGE syndrome pathogenic gene CHD7 mutation detection kit
[0154] 4
[0155] 1
[0156] 23
[0157] DNA
[0158] Synthetic
[0159] 1
[0160] cattgtcttg tccctcttct cta 23
[0161] 2
[0162] 23
[0163] DNA
[0164] Synthetic
[0165] 2
[0166] ggagacagga atgactatac acc 23
[0167] 3
[0168] 23
[0169]DNA
[0170] Synthetic
[0171] 3
[0172] ccctcttctc tatcttccct atg 23
[0173] 4
[0174] 24
[0175] DNA
[0176] Synthe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com