A kit for in vitro detection of the c.602deltg mutation of the neurofibromastosis 2 disease-causing gene NF2
A kit and disease technology are applied in the field of kits for detecting c.602delTG mutation of NF2 gene, which can solve the problems of NF2 disease screening methods or kits that have not been reported, and achieve simple diagnostic methods, ease social pressure, and detect direct result
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
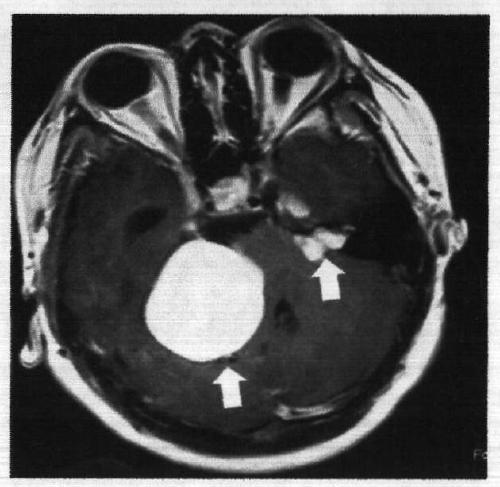
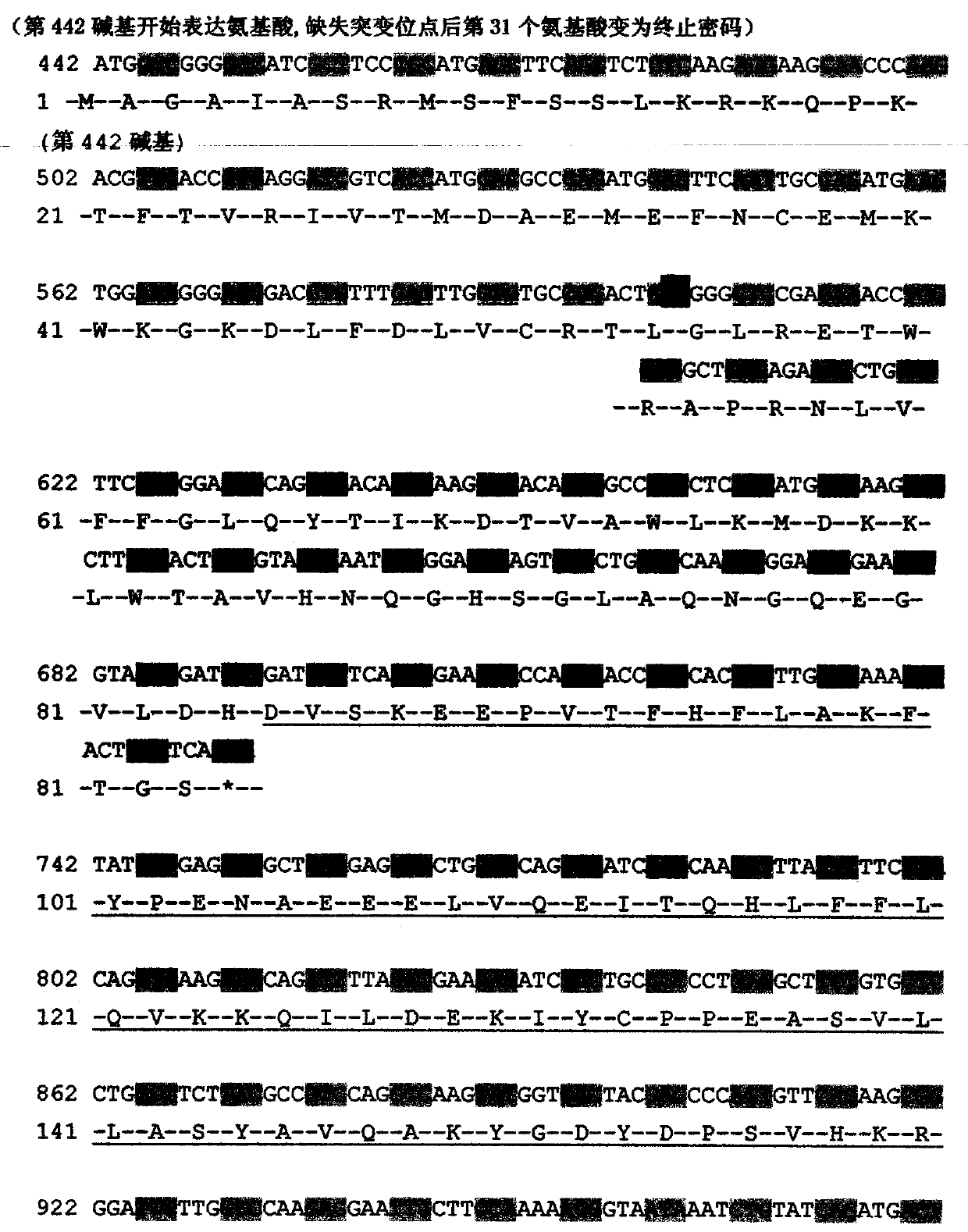
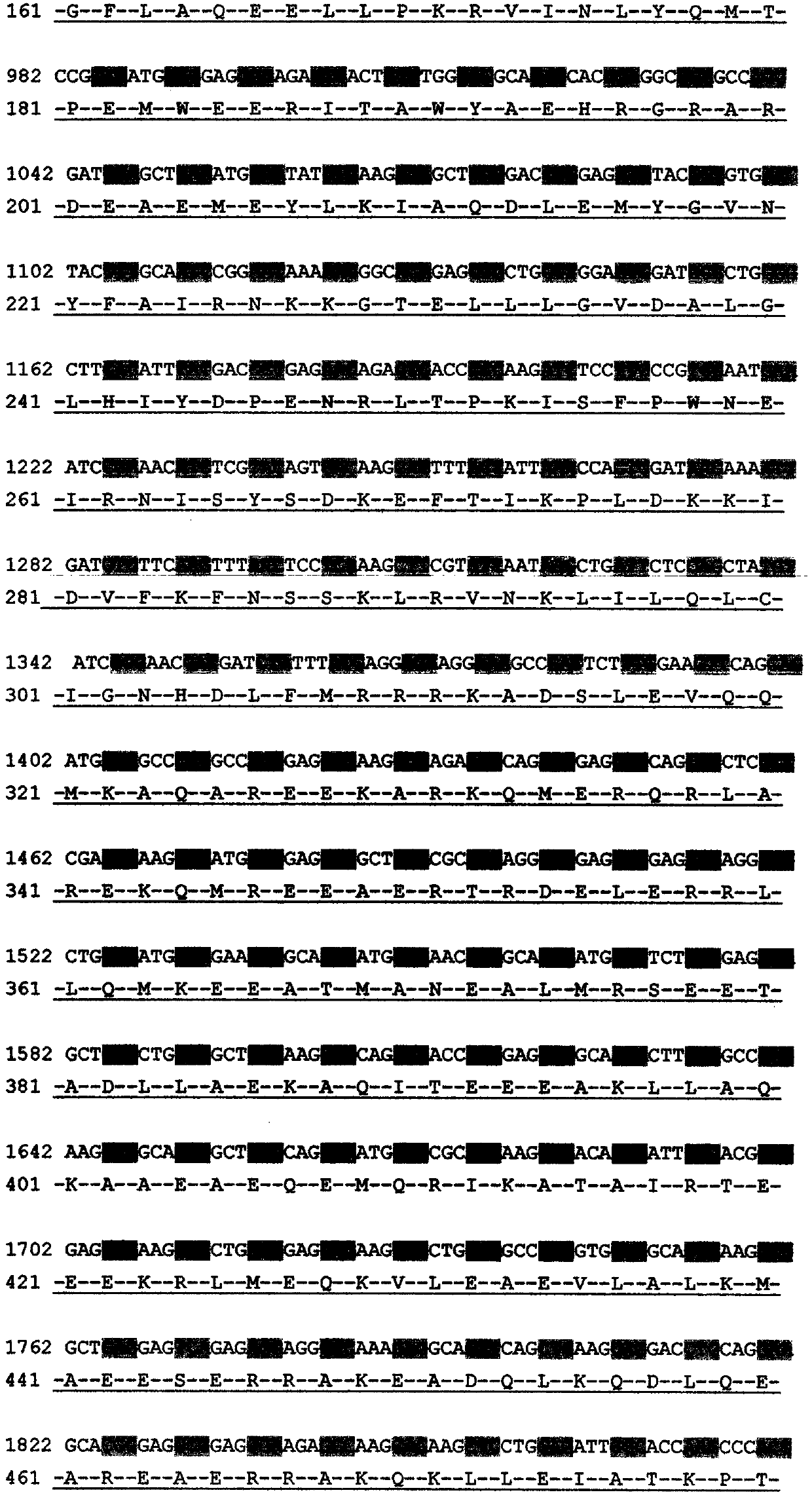
[0017] Example 1. Discovery of c.602delTG at NF2 gene mutation site related to neurofibromatosis type 2 disease
[0018] Collect NF2 patients through the neurosurgery outpatient department. After the patients and their families sign the informed consent form voluntarily, 5-10ml blood samples are collected to establish an inpatient medical record database to record the patient's condition, the incidence of the family and the contact information in detail. Then, phenol-chloroform extraction method was used to extract genomic DNA, quantified, stored in the warehouse, and stored at -20°C. Each piece of DNA accurately corresponds to the clinical data of the registered patient. Design primers according to Primer Premier5.0, including NF2 gene 602 site and both sides of the sequence, for PCR amplification. The PCR products are directly sequenced. The sequencing primers are the same as the PCR amplification primers, and the ABI 3730 DNA sequencer is used for reverse sequencing. Align th...
Embodiment 2
[0020] Example 2. Diagnostic kit for NF2 type disease and its application
[0021] 1. Kit composition
[0022] Upstream primer: NF2602-F: 5'-CCTTCCCCATTGGTTTGTTATTG-3' (SEQ ID NO: 1);
[0023] Downstream primer: NF2602-R: 5'-CCAGGGCCAGCAGCAGTCTAATC-3' (SEQ ID NO: 2);
[0024] 50ul 10X PCR buffer (Pharmacia),
[0025] 10ul 10mM dNTP mixture (Pharmacia),
[0026] 5ul (5unit / ul) Taq DNA polymerase (Takara),
[0027] Each 10ul (10pmol / ul) F1 (SEQ ID NO: 1) and R1 (SEQ ID NO: 2) primers,
[0028] 1ml pure water (homemade).
[0029] 2. Kit application
[0030] (1) Diagnostic materials:
[0031] Peripheral blood samples of 36 patients with NF2 disease and 6 normal persons.
[0032] Collect NF2 patients through the neurosurgery outpatient department. After the patients and their families sign the informed consent form voluntarily, 5-10ml blood samples are collected to establish an inpatient medical record database to record the patient's condition, the incidence of the family and the contact informati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com