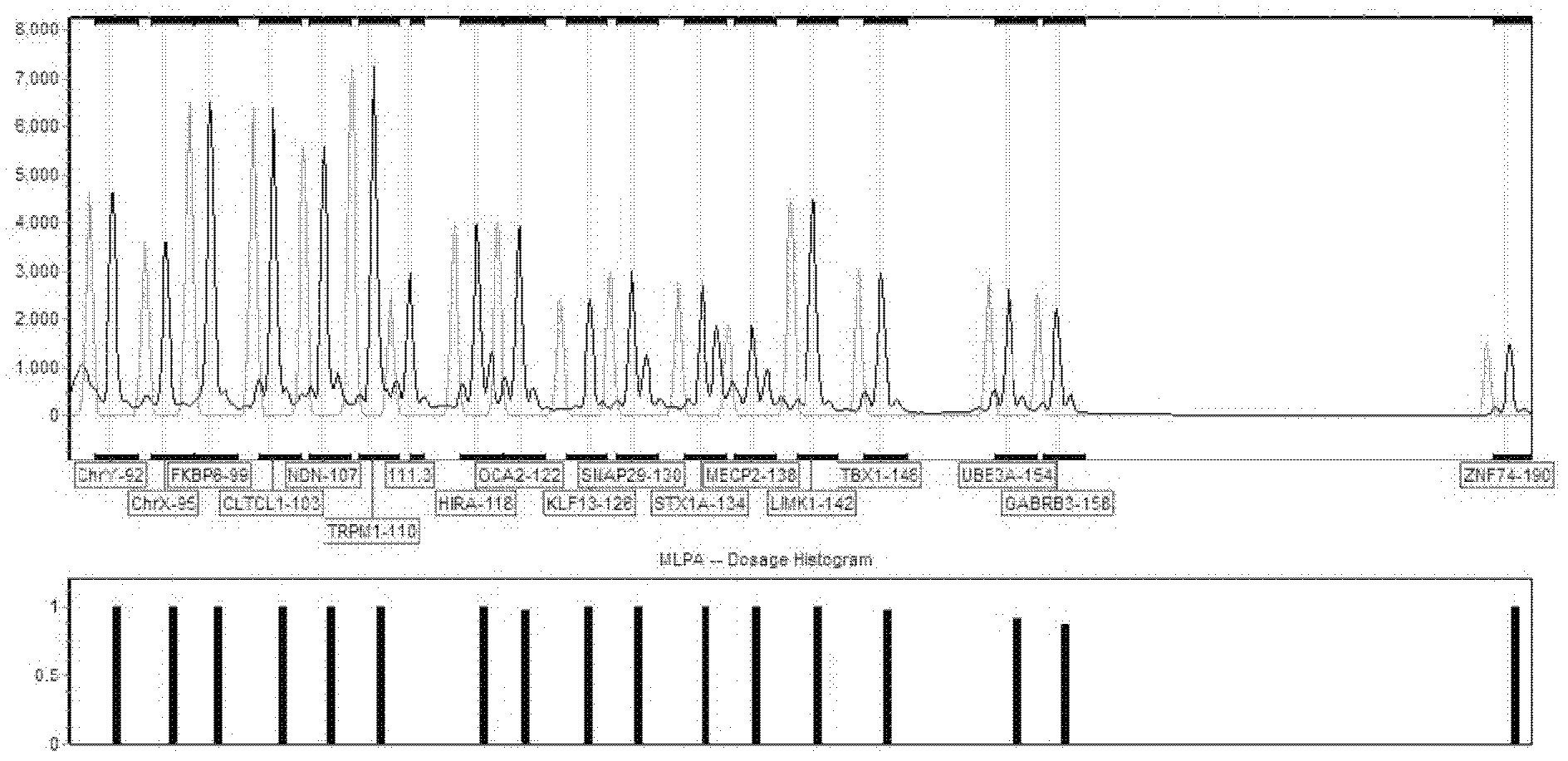
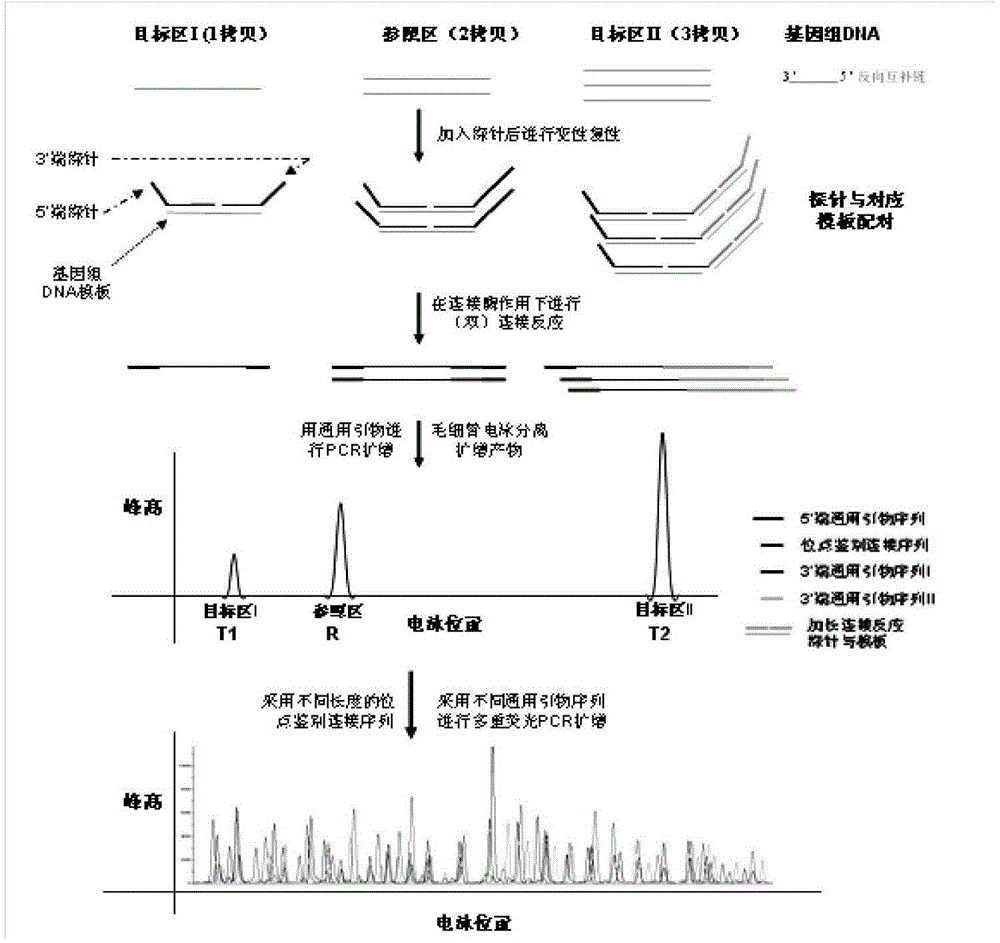
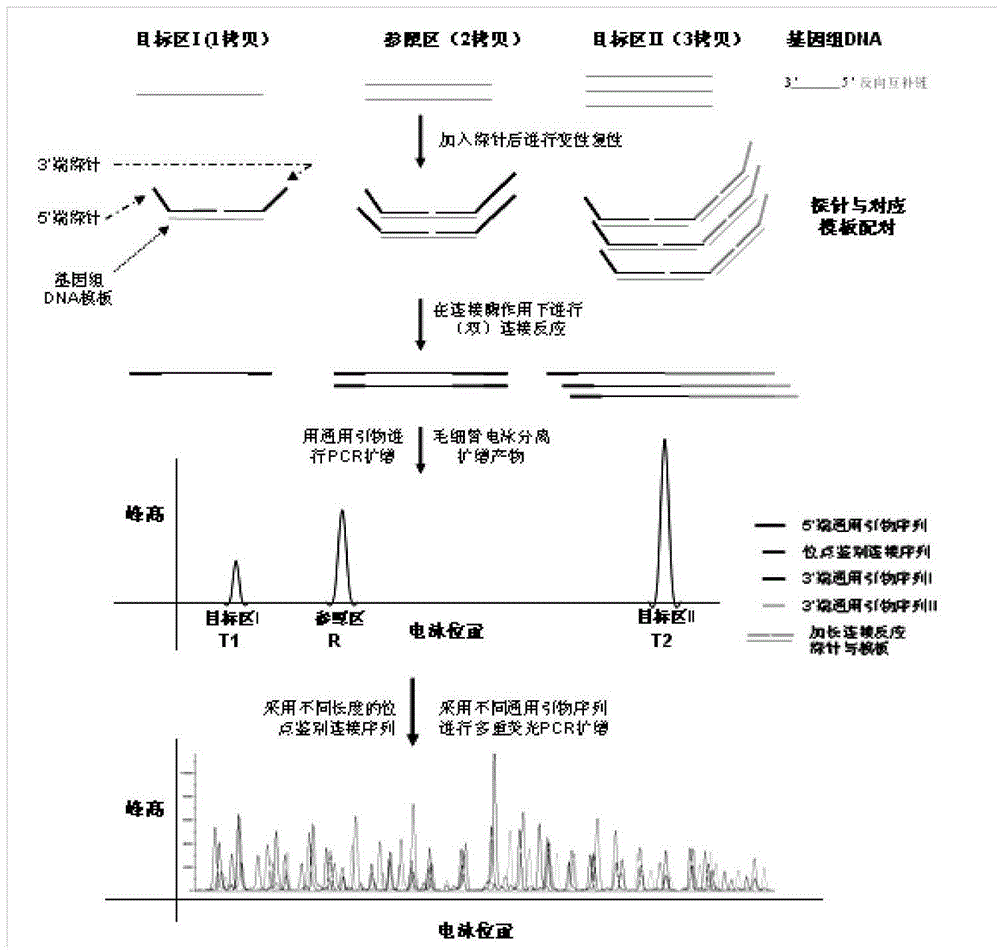
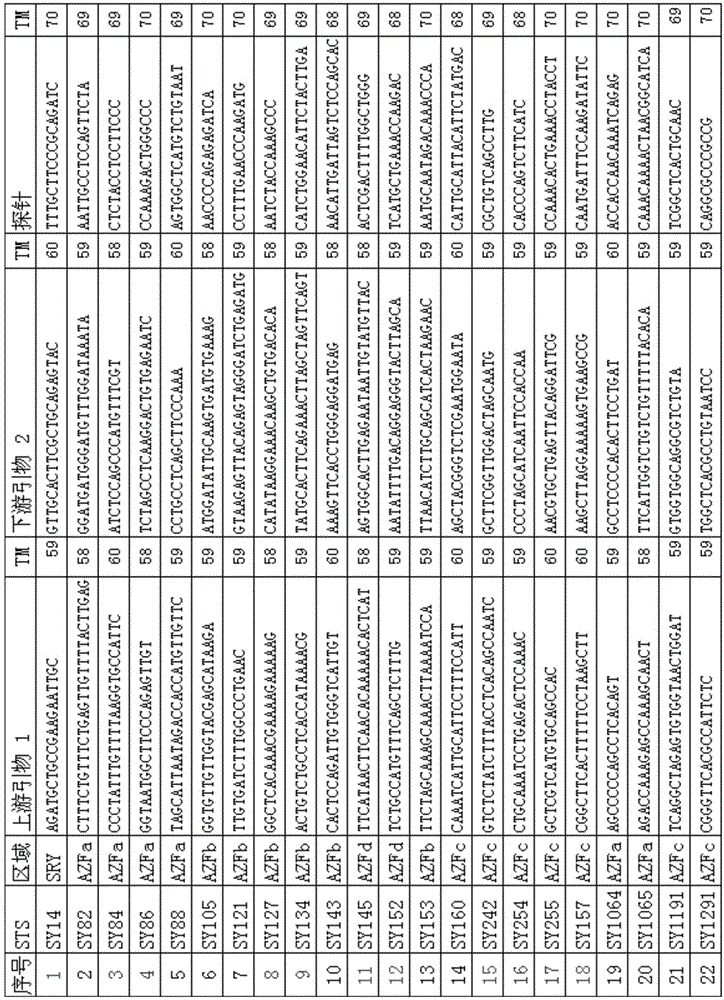
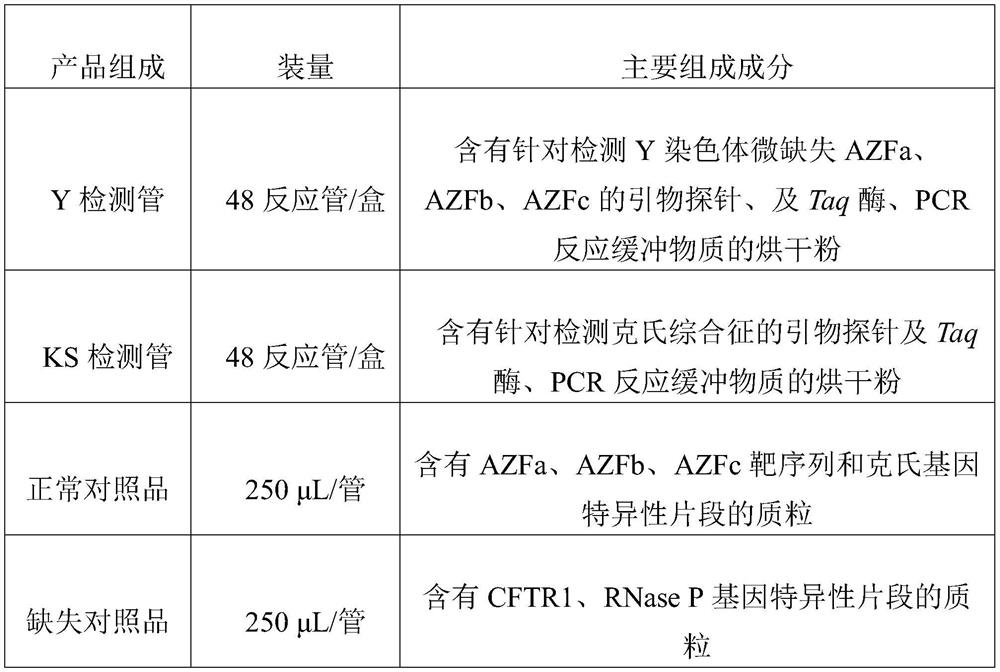
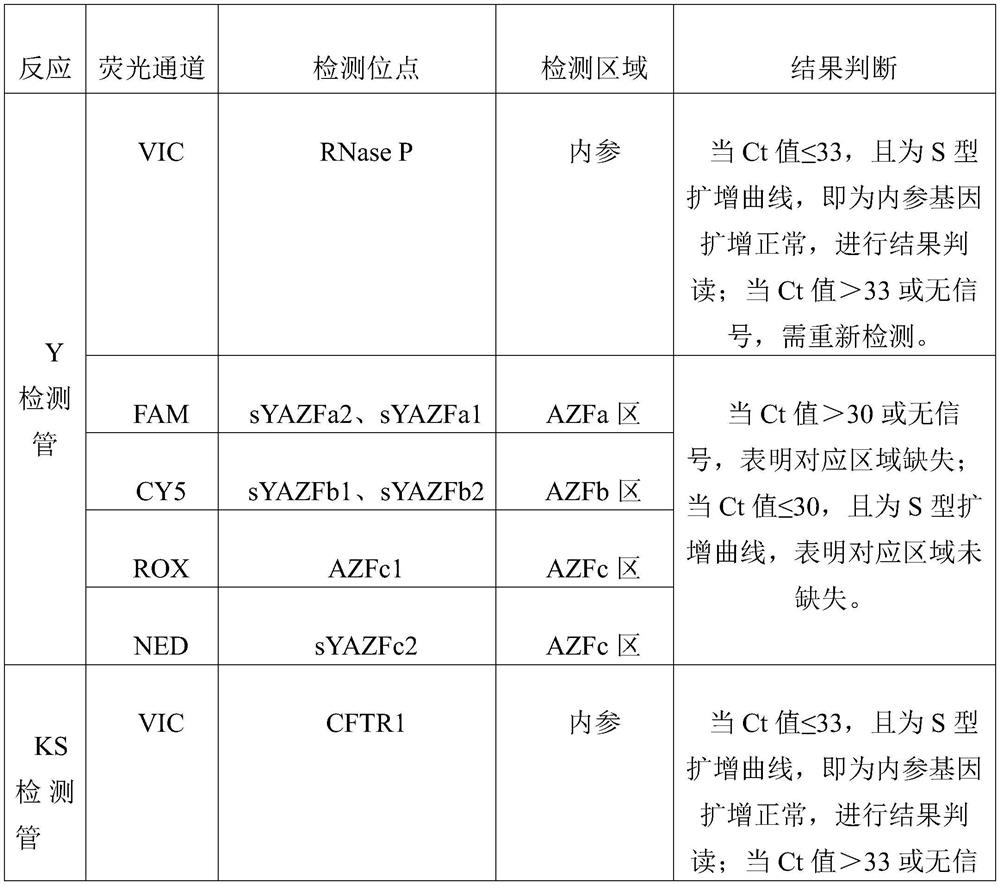
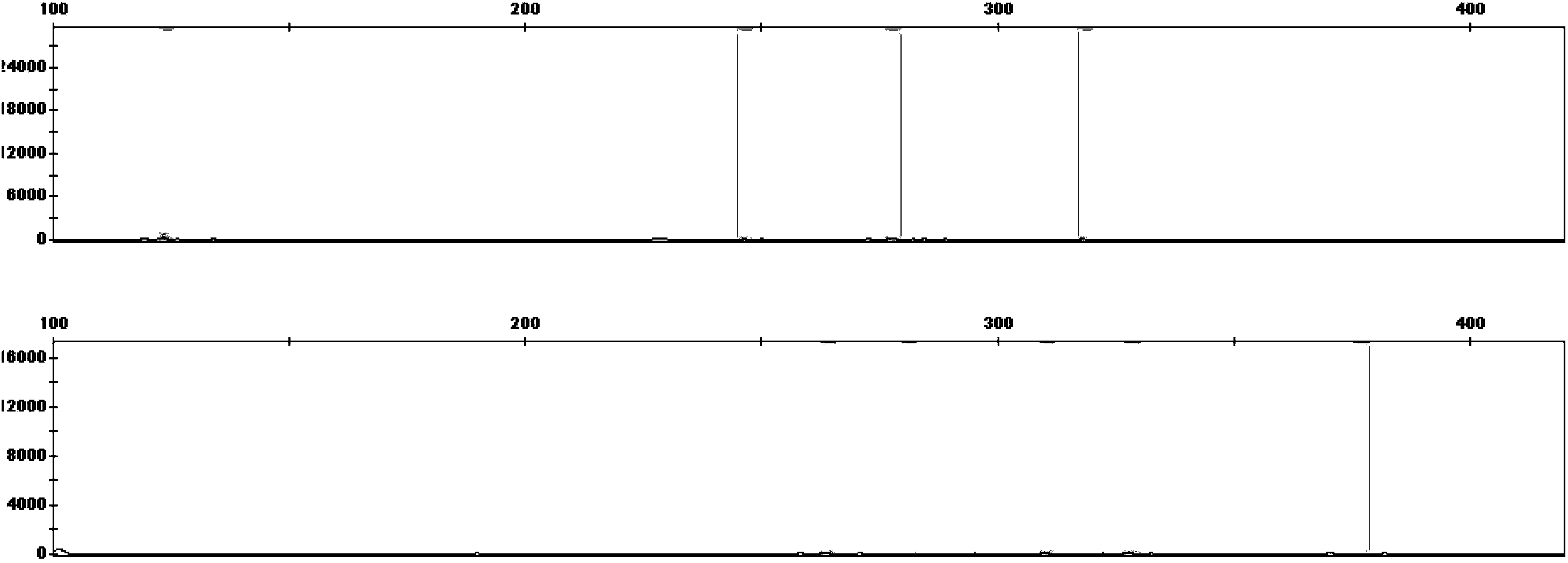
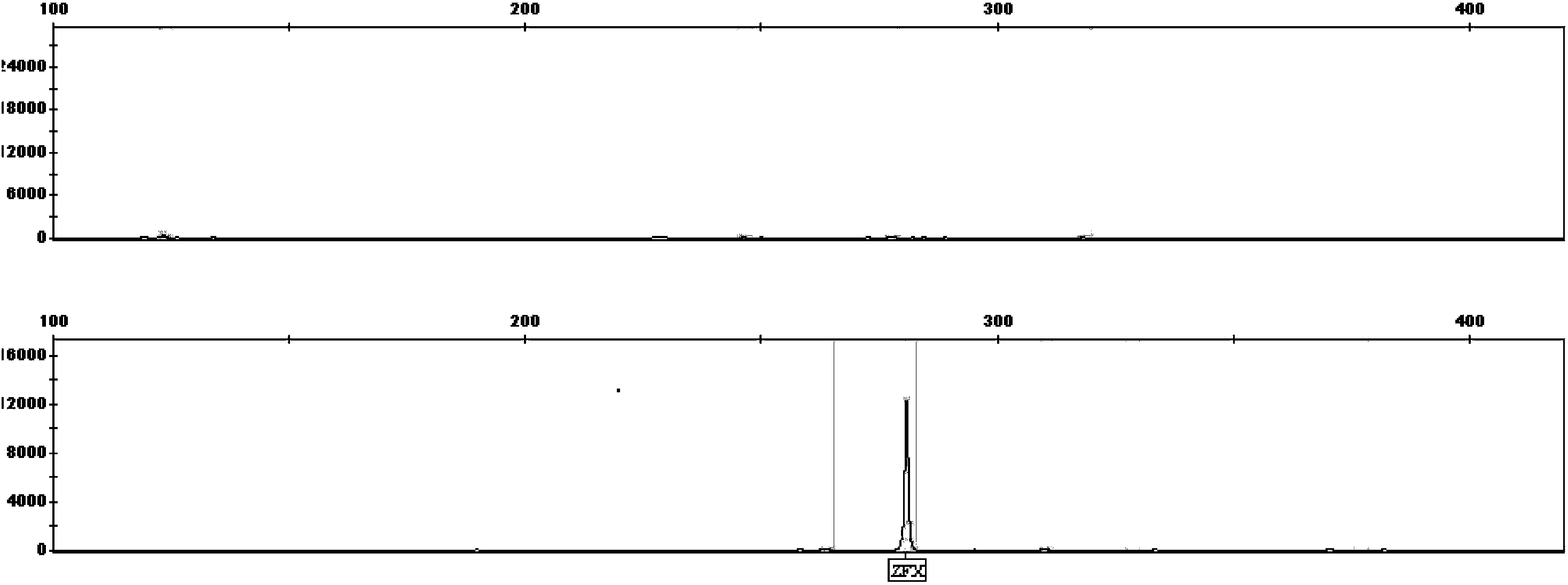
Patents
Literature
38 results about "Chromosome microdeletion" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Amplification composite for detecting microdeletion of Y-chromosome and detection kit
ActiveCN102912028AReliable resultsThe test results are credibleMicrobiological testing/measurementDNA/RNA fragmentationFluorescenceFluorescent pcr
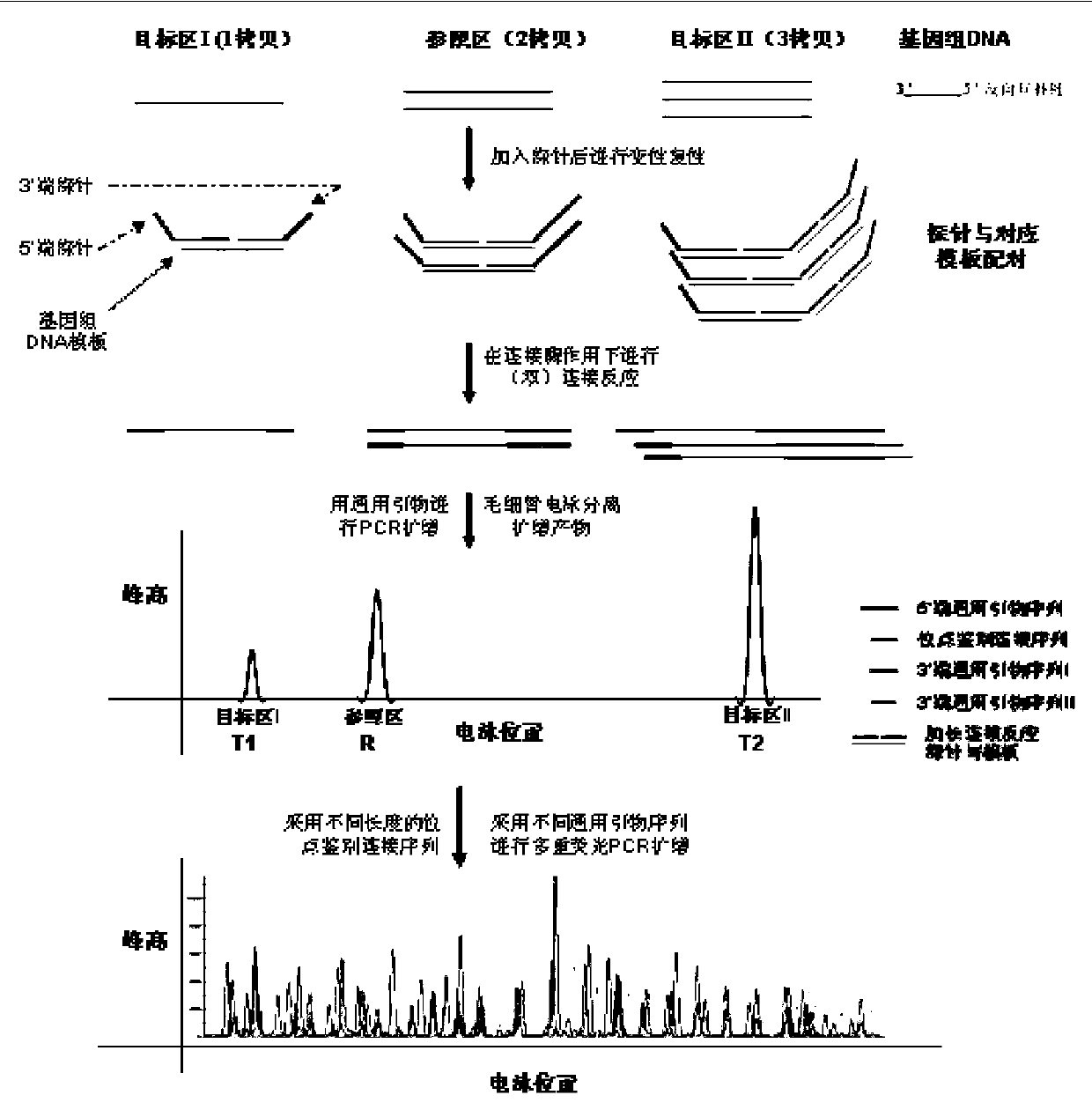
The invention relates to amplification composite for detecting the microdeletion of Y-chromosome and a detection kit, belonging to the field of biotechnical detection. The amplification composite for detecting the microdeletion of Y-chromosome can be amplified to as many as 30 sites related to the microdeletion detection of the Y-chromosome through one reaction. The detection kit detects the microdeletion of the Y-chromosome through the quantitative fluorescent PCR (Polymerase Chain Reaction) method by using the amplification composite. The microdeletion abnormality of the Y-chromosome is determined according to the existence of an amplification product and the quantity of the amplification product. A large quantity of sites enables the detection result to be more convincible and can provide much more and more detailed information for determining the deletion type, and the quantitative detection of partial deletion and repetition can be realized. The detection kit is easier and more convenient to operate, only one PCR amplification and one sequencer detection reaction are needed to complete the detection of one sample, the whole process needs 4-5 h, and the operation intensity and the detection time are greatly reduced.
Owner:BEIJING MICROREAD GENE TECH
Method for detecting chromosome microdeletion and micro-duplication of human embryo
ActiveCN104745718AHigh sensitivityLow costMicrobiological testing/measurementEmbryoChromosome microdeletion
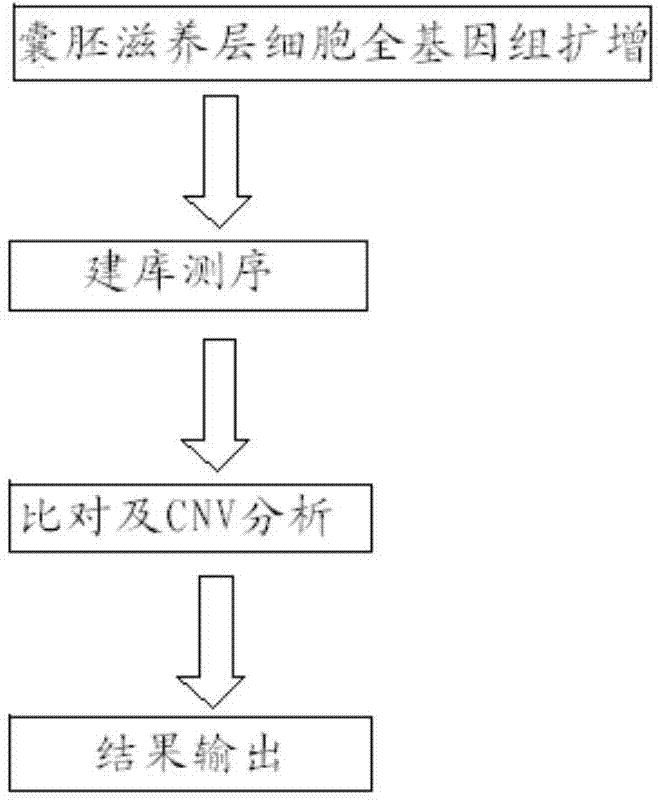
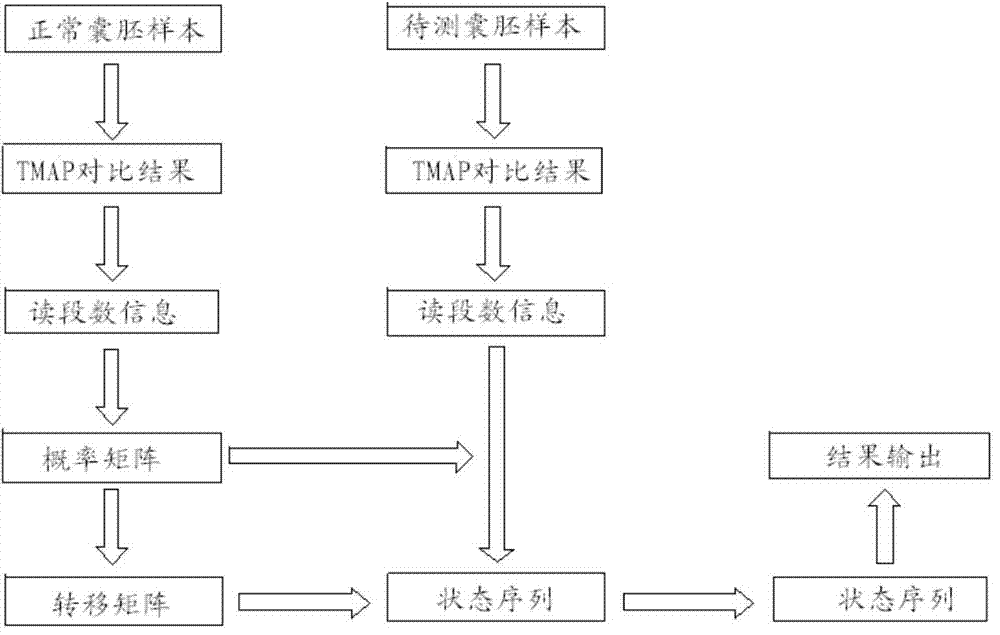
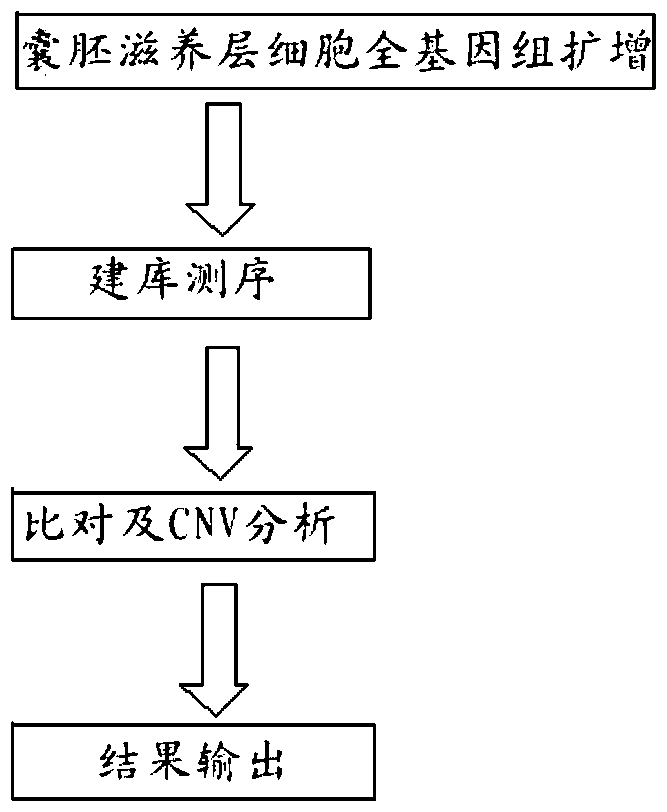
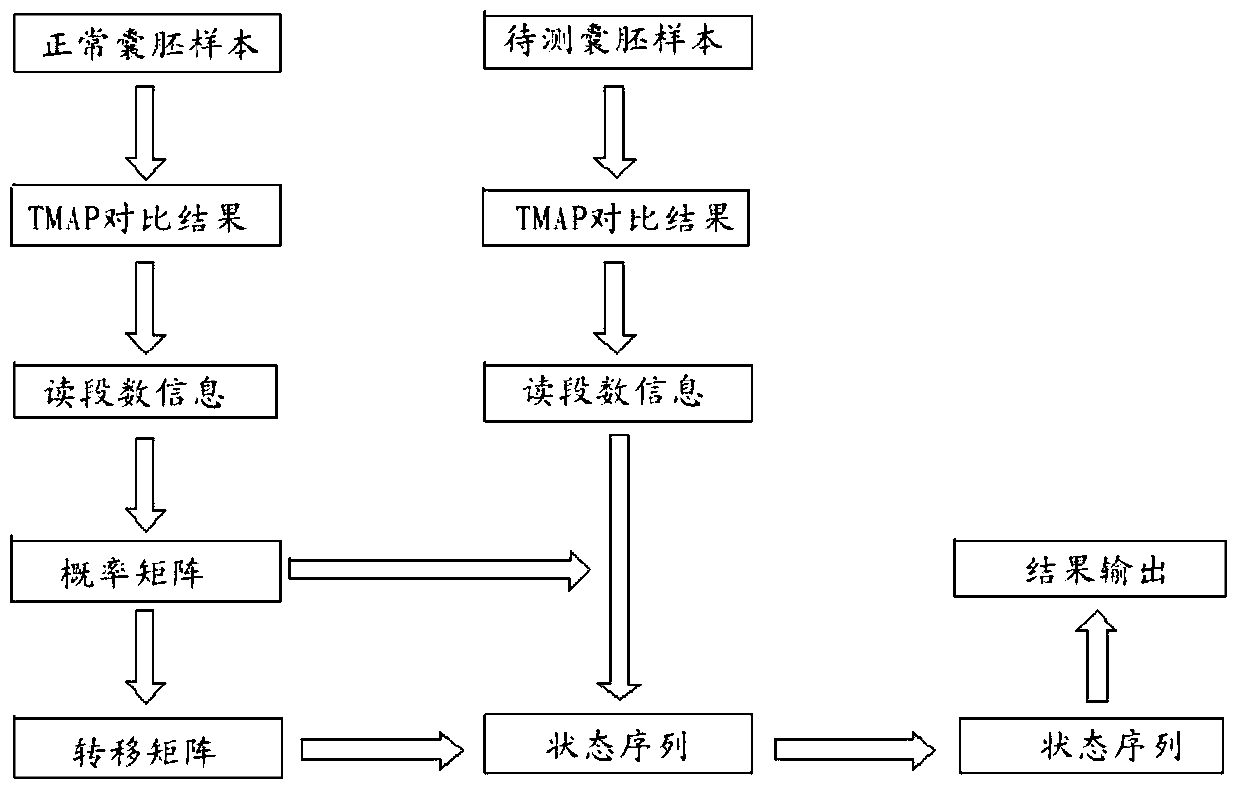
The invention relates to a method for detecting chromosome microdeletion and micro-duplication of a human embryo. The method comprises the following steps of performing whole genome amplification on cells cultured in vitro, interrupting DNA (deoxyribonucleic acid) molecules, and sequencing DNA fragments to obtain sequencing reads; comparing the sequencing reads with a reference sequence, and positioning the sequencing reads on the reference sequence; screening non-repeated areas of the reference sequence, and reserving the non-repeated areas; establishing a matrix of read number in windows through normal samples, analyzing the data of the normal samples, performing statistics on the read number of all the windows in the non-repeated areas, and establishing a probability matrix of the read number and chromosome enpeoids; calculating the copy number, i.e., the A / B / C state, of loci; selecting m continuous loci, i.e., the A state, as micro-duplication loci, and selecting m continuous loci, i.e., the C state, as microdeletion loci; contrasting the micro-duplication loci and the microdeletion loci with the existing CNV (copy number variation) and disease database, performing basic gene annotation and gene function analysis which relates to deletion parts, and annotating with a microdeletion syndrome disease type.
Owner:BEIJING ZHONGYI KANGWEI MEDICAL INSTR
Molecular combination probe for diagnosing and screening chromosome microdeletion syndrome
InactiveCN103014142AEconomic screeningQuick screeningMicrobiological testing/measurementDNA/RNA fragmentationBiotechnologyPhosphorylation
The invention belongs to the technical field of biology, and particularly relates to a molecular combination probe for diagnosing and screening chromosome microdeletion syndrome. The molecular combination probe is used for selecting the key gene of the Williams syndrome, the 22q11 microdeletion syndrome, the Prader-Willi syndrome, the Angelman syndrome, the 15q13.3 microdeletion syndrome and the Rett syndrome, or the gene within a critical area, or the gens arranged at two ends within a duplication / deletion fragment, selecting the sequence which meets a corresponding condition as a probe sequence according to the sequence of the gene, and adding a general primer sequence and adding a phosphorylation mark to the 5'end of a probe left-half sequence and the 3'end of a right-half probe to prepare the combination probe for the multiple continuous probe amplification technology. According to the combination probe provided by the invention, the defects of the fluorescent quantitative PCR (polymerase chain reaction) can be overcome, a plurality of sequences can be analyzed for once, and the molecular combination probe is higher in resolution ratio, sensitivity and repeatability. The probe can be used for the clinical molecular diagnosing and screening of the six-chromosome microdeletion syndrome.
Owner:FUDAN UNIV
Method, kit and analysis system for synchronous prenatal screening of chromosomes and monogenic diseases
ActiveCN111951890AMicrobiological testing/measurementBiostatisticsPrenatal screeningChromosome microdeletion
The invention provides a detection method, a kit and a system for noninvasive prenatal screening of fetal chromosome copy number variation, fetal chromosome microdeletion / microduplication and / or dominant single gene mutation. The invention further provides a design method of a targeted capture probe. The detection method is used for non-invasive prenatal screening of fetuses. Compared with existing non-invasive prenatal screening detection methods, the application range of clinical gene detection can be expanded, and the detection accuracy is improved.
Owner:BEIJING BIOBIGGEN TECH CO LTD +1
Novel chromosome microdeletion/microduplication syndrome detection system and kit
ActiveCN104651516ARealize detectionIncreased length rangeMicrobiological testing/measurementDECIPHERChromosome microdeletion
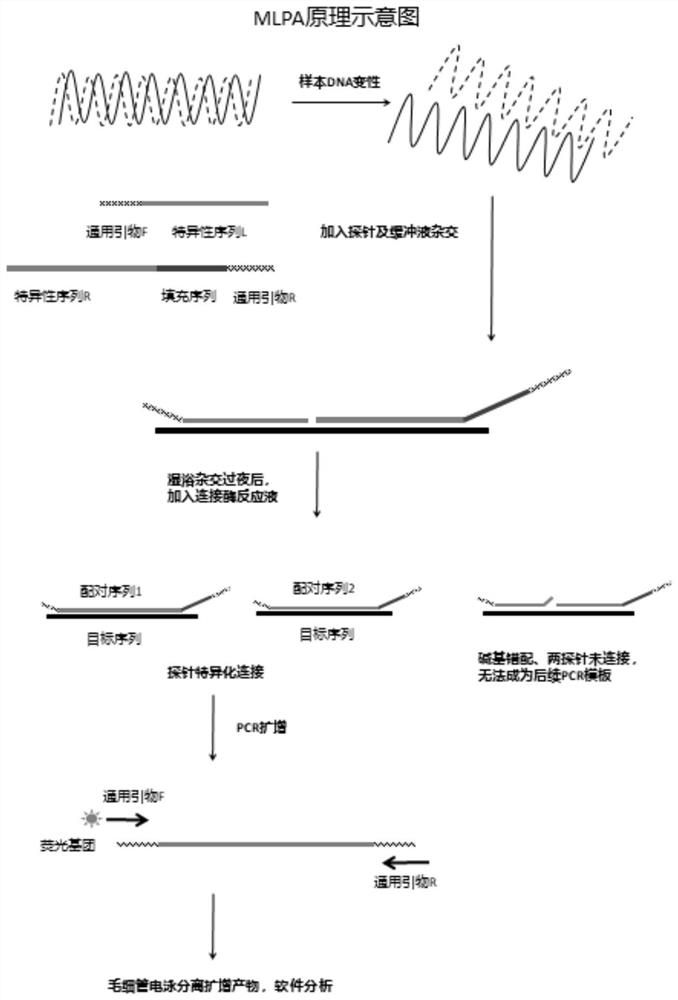
The invention discloses a novel chromosome microdeletion / microduplication syndrome detection system which mainly comprises 643 pairs of probes, multiplex PCR (polymerase chain reaction) amplification primers and correlation detection reaction reagents. The detection process comprises the following steps: hybridizing a probe set with target nucleic acid in the sample; connecting the hybridized probe; amplifying the connecting probe; and detecting the amplification product to determine the existence or quantity of the target nucleic acid in the sample. The platform can implement systematic screening and detection on 24 chromosome aneuploids, 24 chromosome telomere abnormities, more than 70 chromosome microdeletion / microduplication syndromes and multiple monogenic disease mutation hot spots at one time by using the optimized probe set and reaction system. Compared with the existing like detection techniques (such as MLPA and the like), the detection system covers nearly all definite pathogenic regions with abnormal number of copies in the DECIPHER database, has the advantages of wide detection range, high detection precision and low cost, and is simple to operate.
Owner:SUZHOU MUNICIPAL HOSPITAL +1
Y chromosome microdeletion multiple real-time fluorescence PCR detection kit, amplimer pairs and probes
InactiveCN105018589AQuick checkComprehensive Y chromosome microdeletion informationMicrobiological testing/measurementDNA/RNA fragmentationY chromosome microdeletionFluorescence
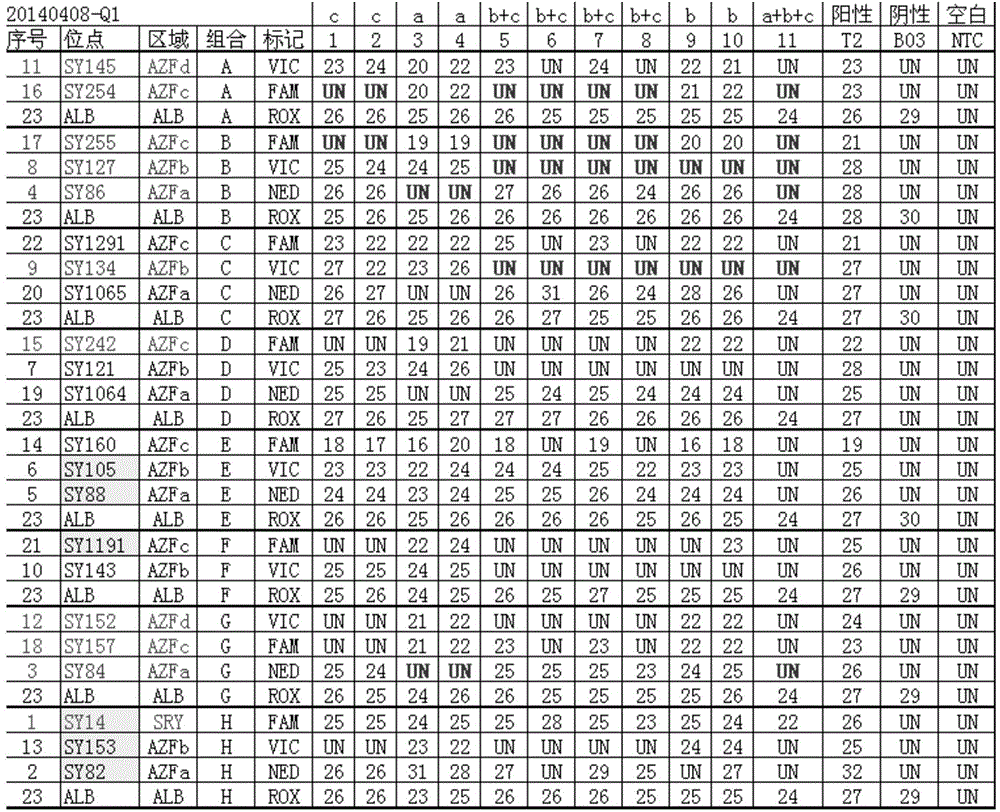
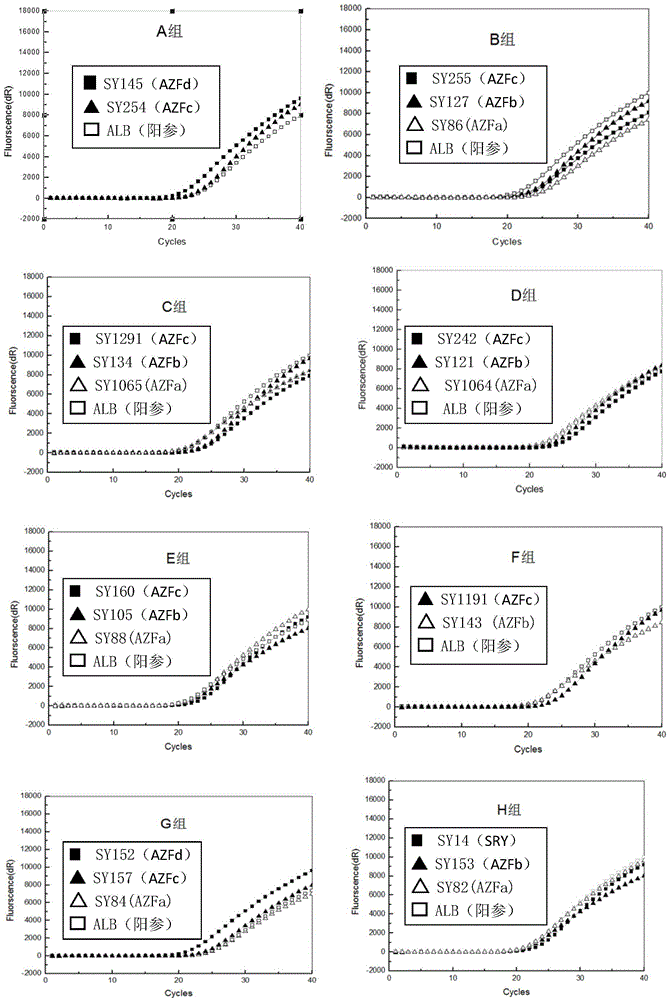
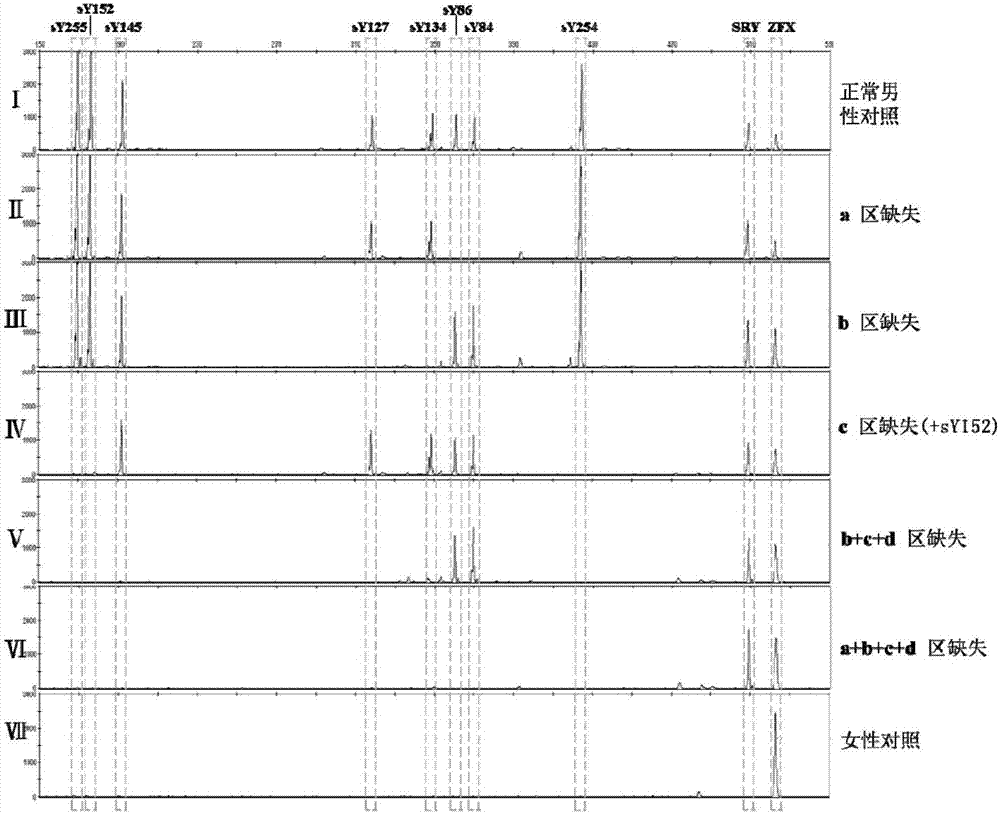
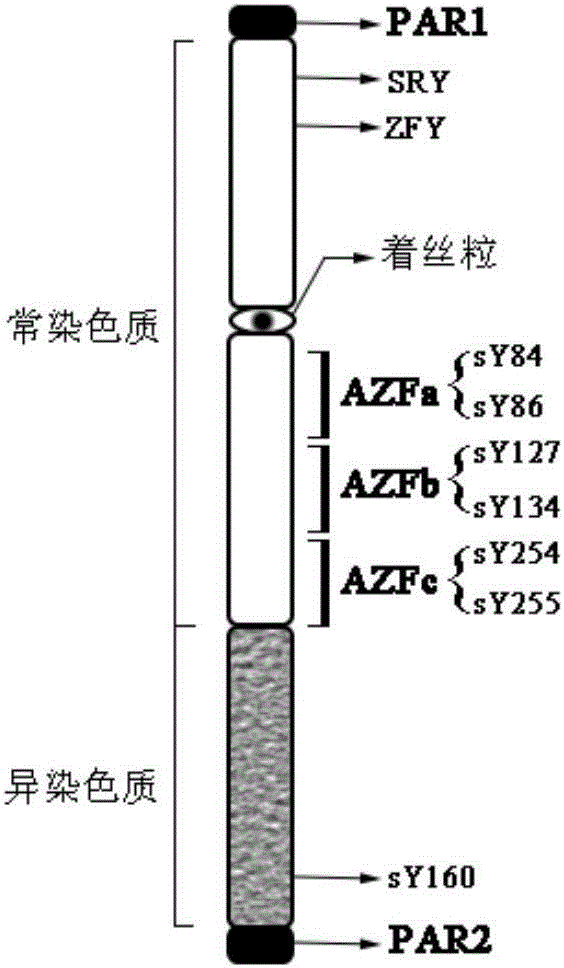
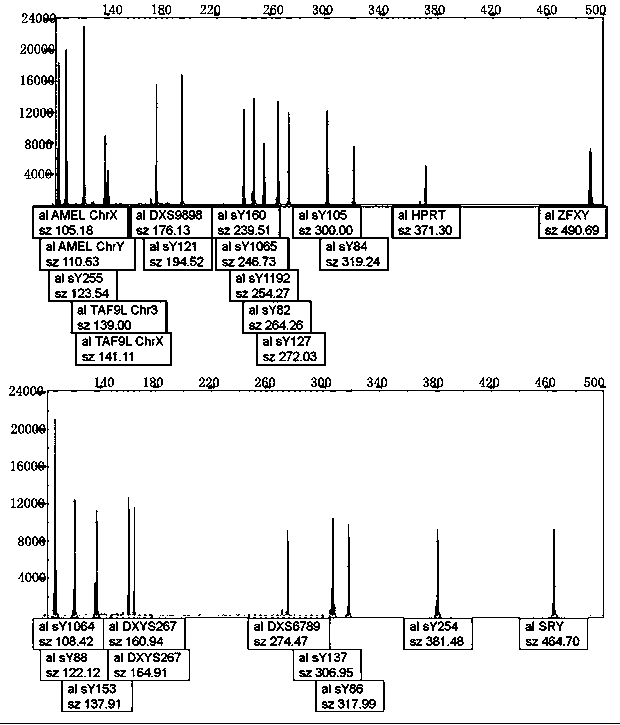
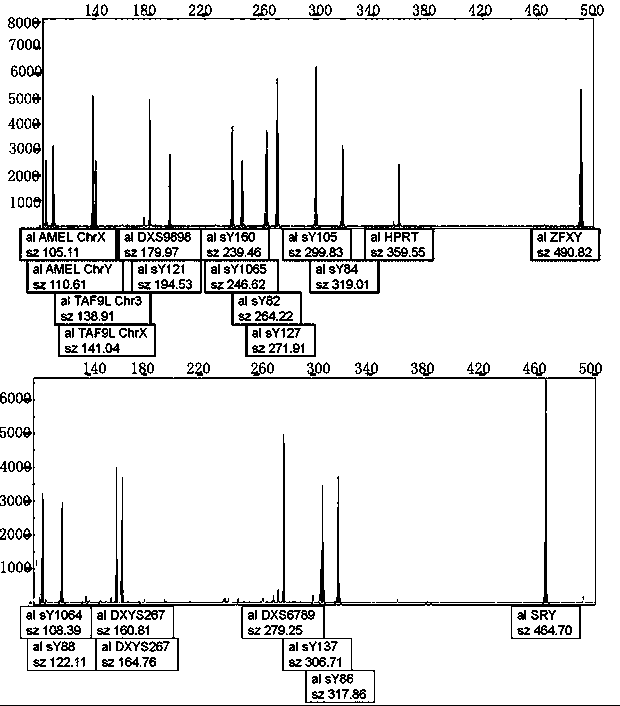
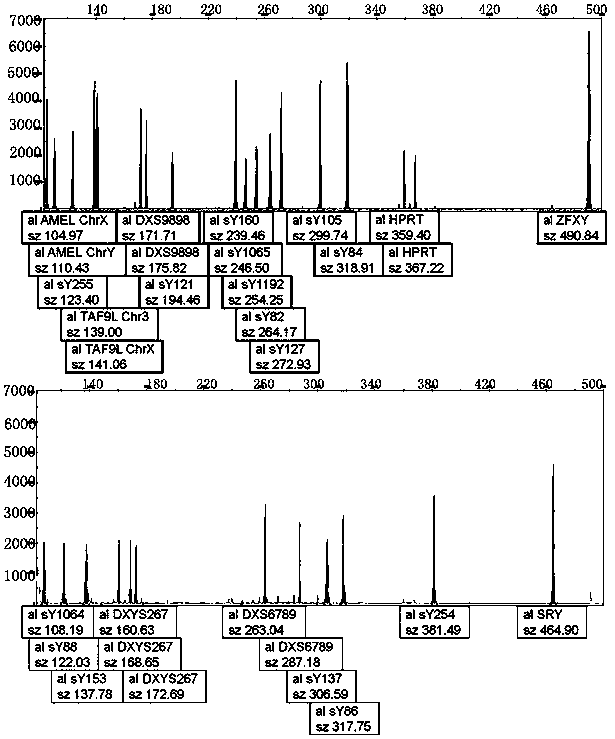
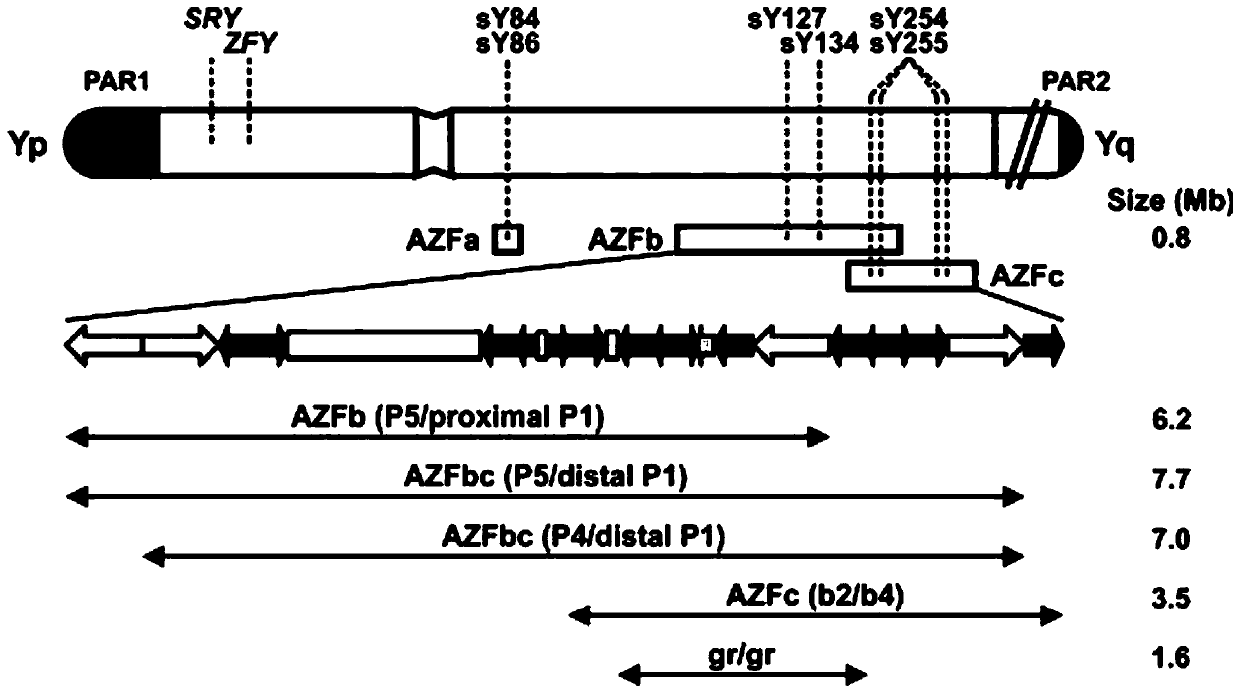
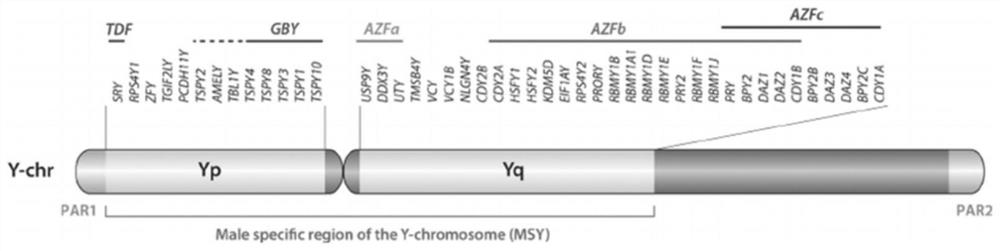
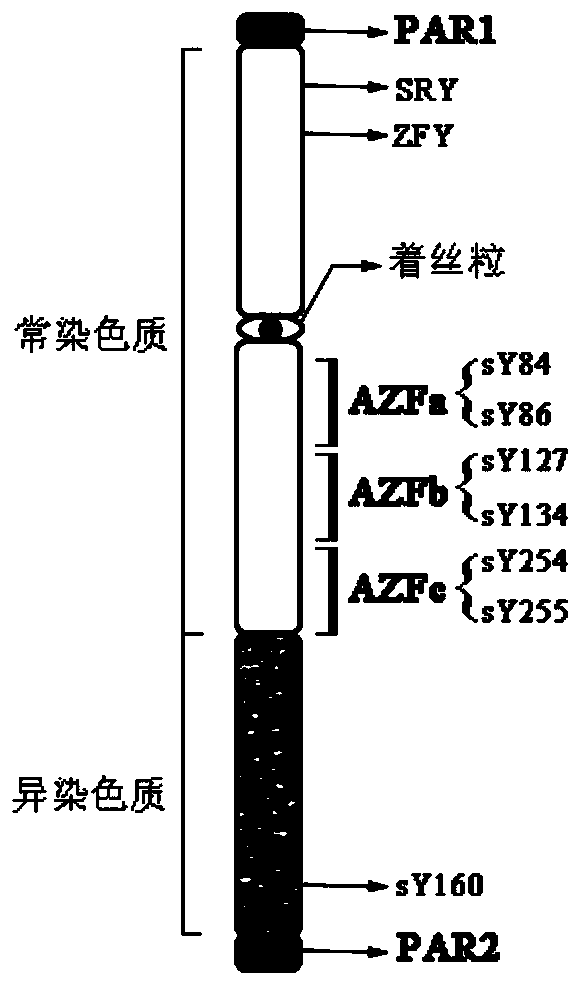
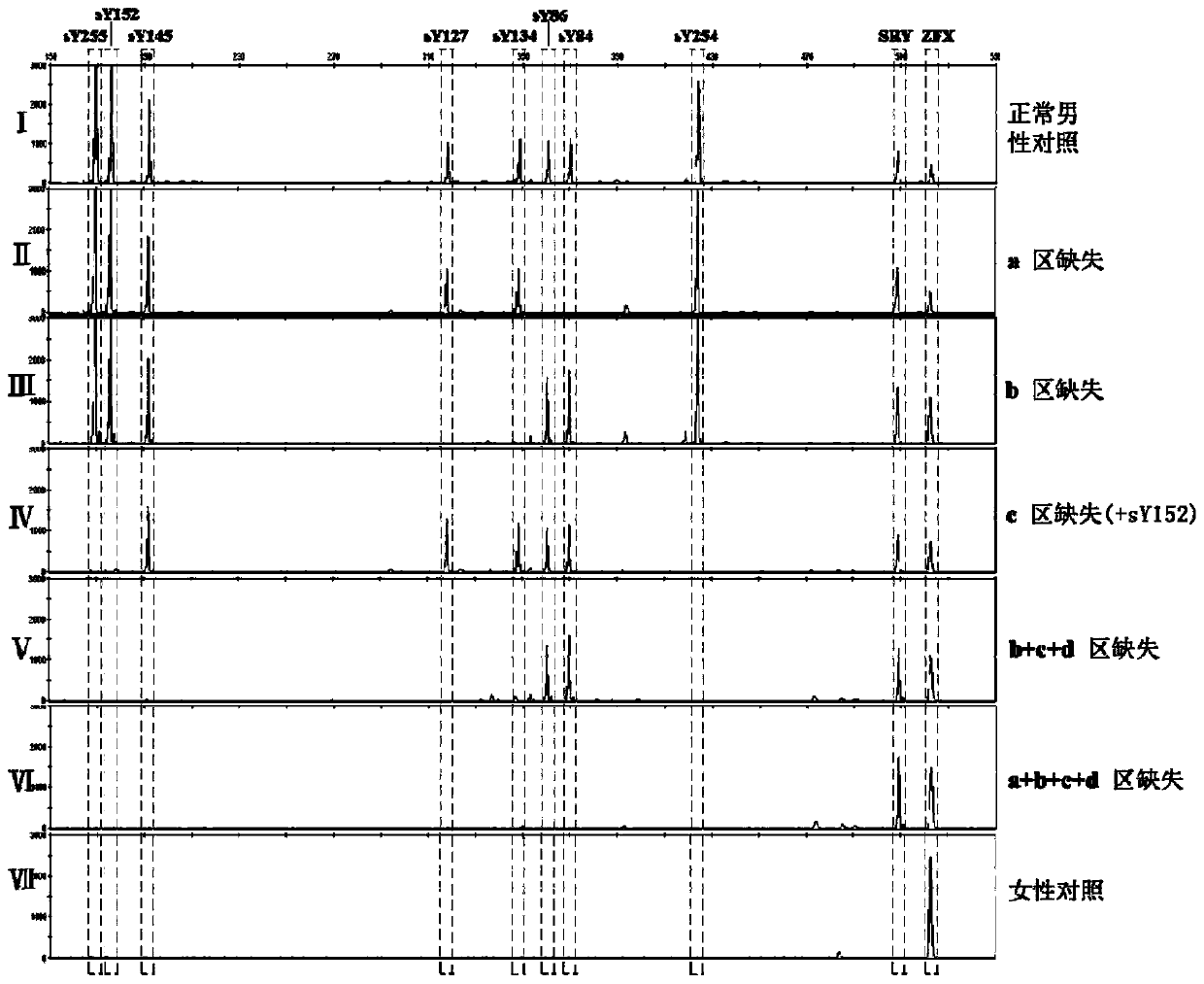
The invention relates to a biomolecule diagnostic reagent and discloses a Y chromosome microdeletion multiple real-time fluorescence PCR detection kit. The Y chromosome microdeletion multiple real-time fluorescence PCR detection kit comprises amplimer pairs and fluorescent probes for detecting SY84, SY86, SY127, SY134, SY254, SY255, SY157, SY242, SY1191 and SY1291, and further comprises amplimer pairs and fluorescent probes for detecting sites including at least one of SY82, SY88, SY1064 and SY1065, at least one of SY105, SY121, SY143 and SY153, SY160 and at least one of SY145 and SY152 for detecting AZFd area deletion. The Y chromosome microdeletion multiple real-time fluorescence PCR detection kit can detect 21 STS sites at most, the deletion coverage rate is larger than 99 percent, a multiple real-time fluorescence quantitative PCR technological platform is adopted, Y chromosome microdelection sites can be quickly detected in a high-throughput mode, whether the AZF a, b, c and d areas are deleted completely or partially is distinguished, and more comprehensive Y chromosome microdeletion information is obtained.
Owner:SHANGHAI HENGJIAN BIOTECH CO LTD
Kit for non-invasive prenatal detection of 12 chromosome microdeletion micro-duplex syndromes and special probe set of kit
The invention discloses a kit for non-invasive prenatal detection of 12 chromosome microdeletion micro-duplex syndromes and a special probe set of the kit. The probe set comprises 600 specific probes.Each specific probe sequentially comprises a DNA fragment 1, a DNA fragment 2 and a DNA fragment 3 from the 5' terminal to the 3' terminal. The nucleotide sequences of the DNA fragments 2 of the 600specific probes are sequentially shown from the 16th position to the 93rd position at the 5' terminal of a sequence 1 of a sequence table to the 16th position to the 93rd position at the 5' terminal of a sequence 600 of the sequence table. Experiments prove that the kit can be used for detecting whether a to-be-detected fetus suffers from the 12 chromosome microdeletion micro-duplex syndromes or not, and detection results are completely consistent with detection results of clinical SNP Aarray. The kit has important application value.
Owner:北京迈基诺基因科技股份有限公司
Multiplex PCR (polymerase chain reaction) primer set for detection Y chromosome microdeletion, kit and application
ActiveCN107012234ALow costEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationMultiplexBase J
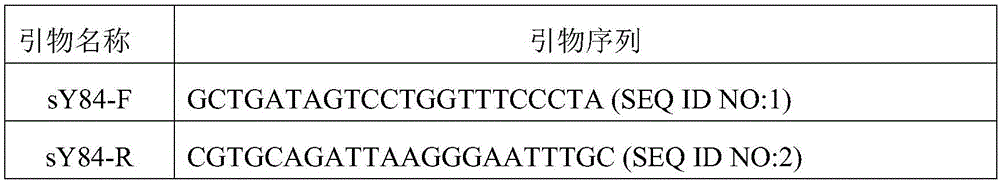
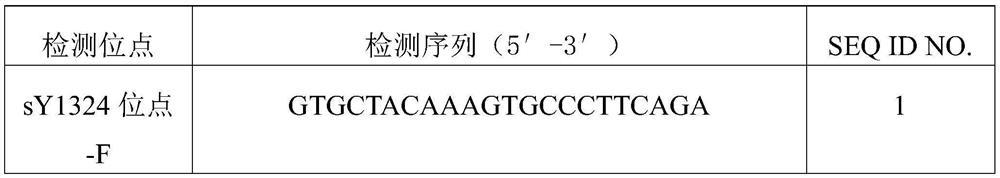
The invention relates to the field of gene deletion detecting, in particular to a multiplex PCR (polymerase chain reaction) primer set for detection Y chromosome microdeletion, kit and application. The multiplex PCR primer set comprises a universal primer and a specific primer, the universal primer has base sequences shown as SEQ ID NO:1 and SEQ ID NO:2, a 5' terminal of the base sequence shown as the SEQ ID NO:1 is marked with a fluorophore, and the specific primer comprises a primer having base sequences shown as SEQ ID NO:3-SEQ ID NO:22. The defect that conventional multiplex PCR is low in sensitivity and non-specific amplification is overcome; through a method combining a novel stable universal primer-multiplex PCR reaction system with a fluorescent labeling universal primer, an amplification product is subjected to capillary electrophoresis analysis, so that high sensitivity of detection is guaranteed, cost is effectively lowered, and large-sample-size efficient detection can be realized.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Product for detecting microdeletion of chromosome 16p11.2
ActiveCN105624308AImprove accuracyEasy to operateMicrobiological testing/measurementSmall probabilityGenotype
The invention discloses a method for detecting microdeletion of 0.6Mb within the area of 29.5-30.1 Mb in a chromosome 16p11.2.According to the detection principle of the method, a small probability event of a genotype homozygous specific product of multiple SNP sites is utilized for judging whether chromosome microdeletion exists or not.The invention also discloses a product for detecting microdeletion of 0.6Mb within the area of 29.5-30.1 Mb in the chromosome 16p11.2.Meanwhile, the product can detect whether an object suffers from congenital scoliosis or not.The detection method and product have a wide clinical application prospect.
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Primer combination and kit used for detecting Y chromosome microdeletion
ActiveCN106591442AIdentify microdeletionsImprove economyMicrobiological testing/measurementDNA preparationY chromosome microdeletionElectrophoresis
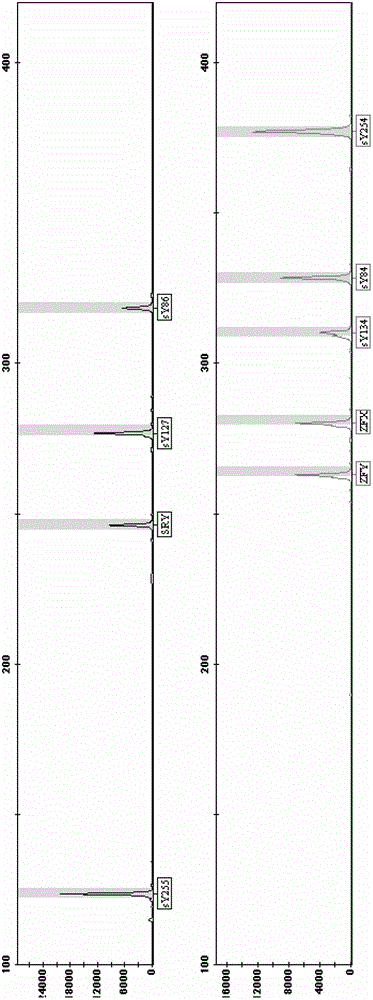
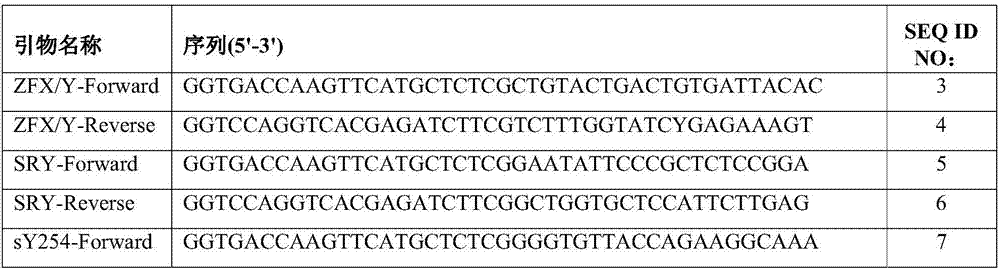
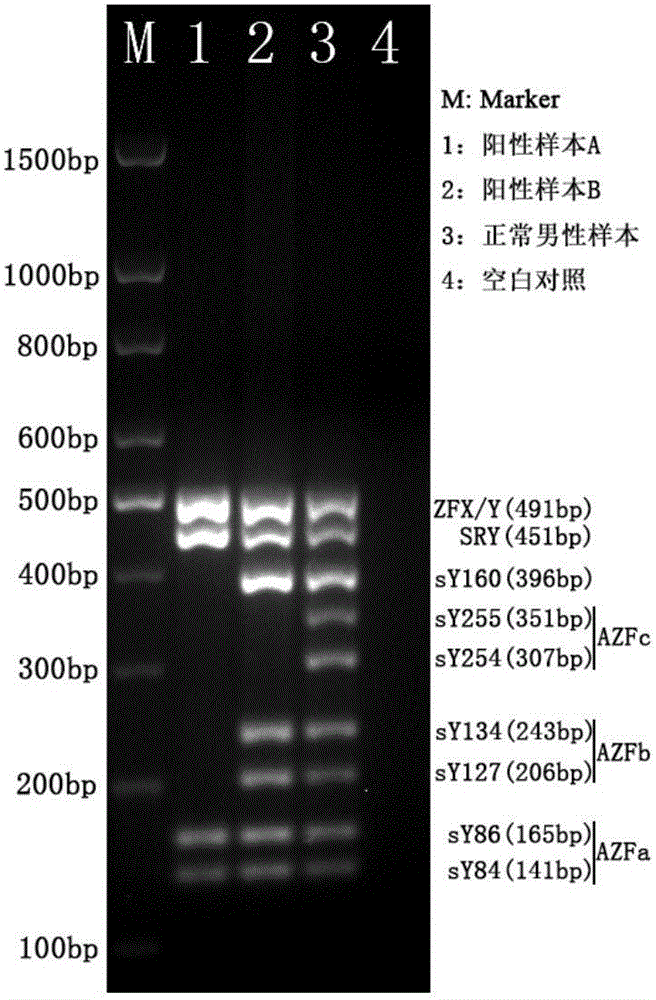
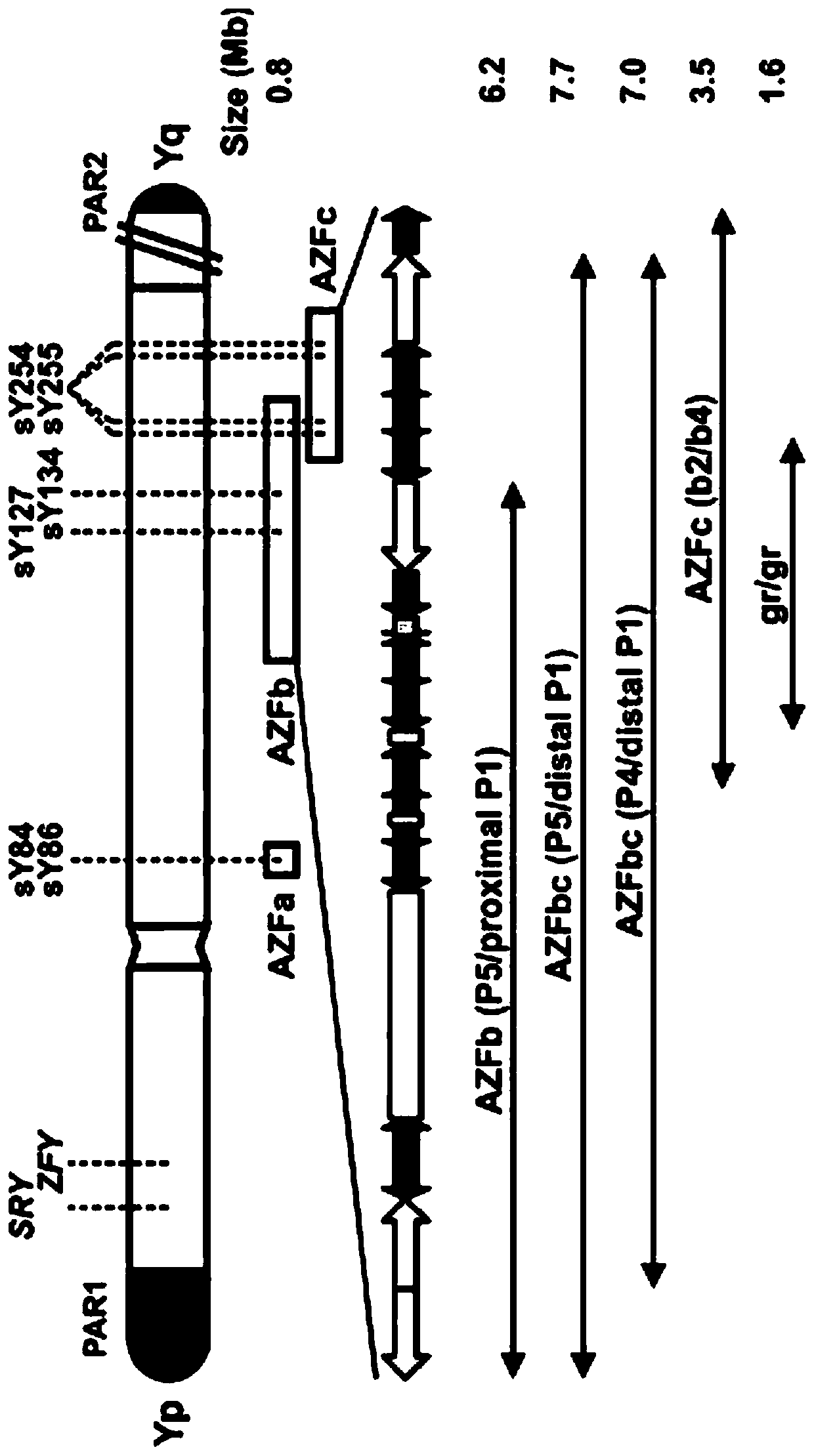
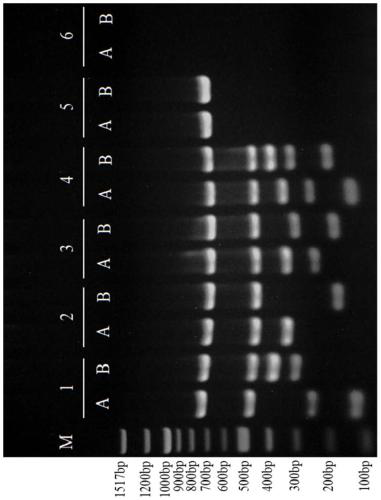
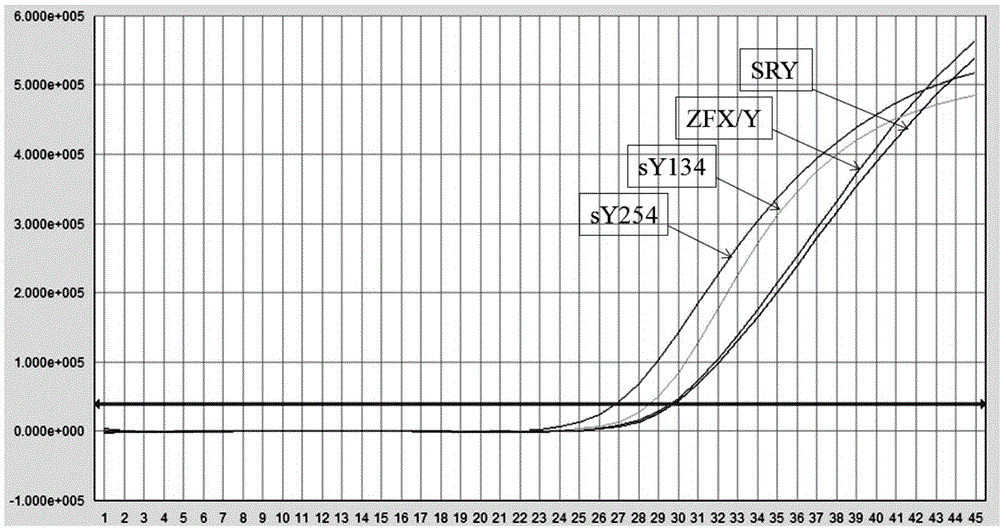
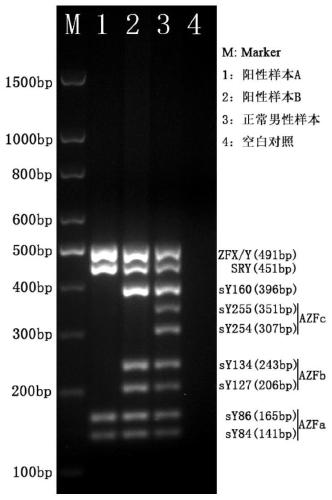
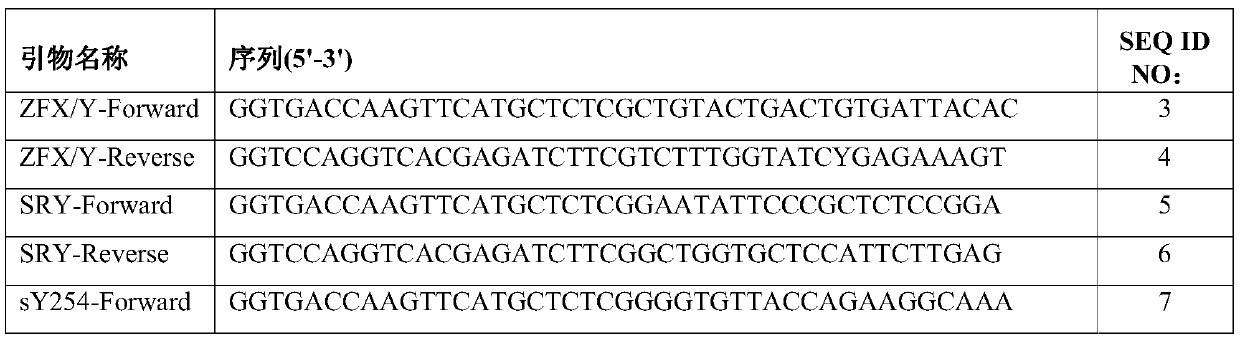
The invention relates to a primer combination and kit for chromosome microdeletion. By the use of a multiple PCR combined with agarose gel electrophoresis technology, seven STS loci closely associated with the Y chromosome microdeletion are detected to judge the microdeletion conditioof a Y chromosome. Seven STS loci with high specificity and sensitivity (sY84, sY86, sY127, sY134, sY254, sY255 and sY160) are chosen as detection loci, at the same time two loci ZFX / Y and SRY are utilized as quality control loci, a primer is designed aiming at each locus respectively, and products of the nine loci after multiplex amplification are directly put on the agarose gel to be conducted electrophoresis detection. By the use of the primer combination and detection system, the Y chromosome microdeletion can be detected only needing single tube amplification reaction.
Owner:北京圣谷智汇医学检验所有限公司
Primer composition for detecting Y chromosome microdeletion and sex chromosome number and application
ActiveCN111471760AIncreased Amplification ConsistencySolving Amplification Consistency IssuesMicrobiological testing/measurementDNA/RNA fragmentationY chromosome microdeletionA-DNA
The invention relates to the field of molecular biology diagnosis, in particular to a primer composition for detecting Y chromosome microdeletion and the sex chromosome number and an application. A Ychromosome AZF region sequence marker site and a sex chromosome number detection site can be simultaneously amplified by utilizing special primer design in a tube of PCR reaction solution; a male sexdetermination gene (SRY) is used as a sex abnormality control and a human X / Y linked zinc finger protein gene (ZFX / Y) is used as an internal control; PCR products are analyzed through capillary electrophoresis; and whether an AZF region of Y chromosome is deleted or not and whether the sex chromosome number is abnormal or not are interpreted by comparing the PCR products with different fragment sizes. A result can be obtained within 3 hours from a DNA sample, the Y chromosome microdeletion and the Klinefelter syndrome can be detected at the same time only through one PCR experiment, the detection technical process is simple, and standardization is easy to achieve.
Owner:XIAMEN BIOFAST BIOTECHNOLOGY CO LTD
Composition and product for detecting Y chromosome microdeletion, application of composition and method
PendingCN111778320ASimple and fast operationShorten the timeMicrobiological testing/measurementBiotechnologyY chromosome microdeletion
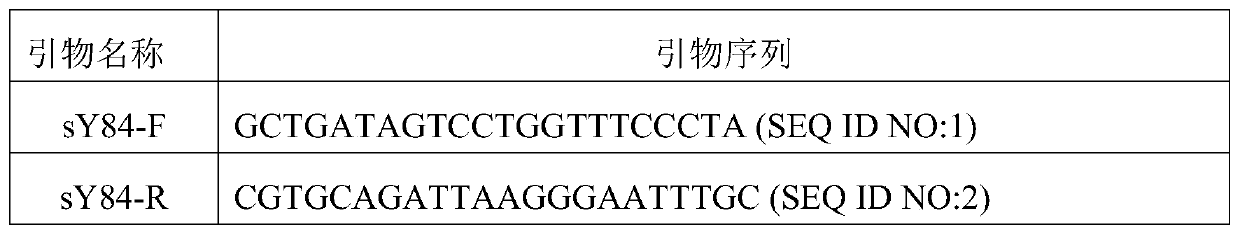
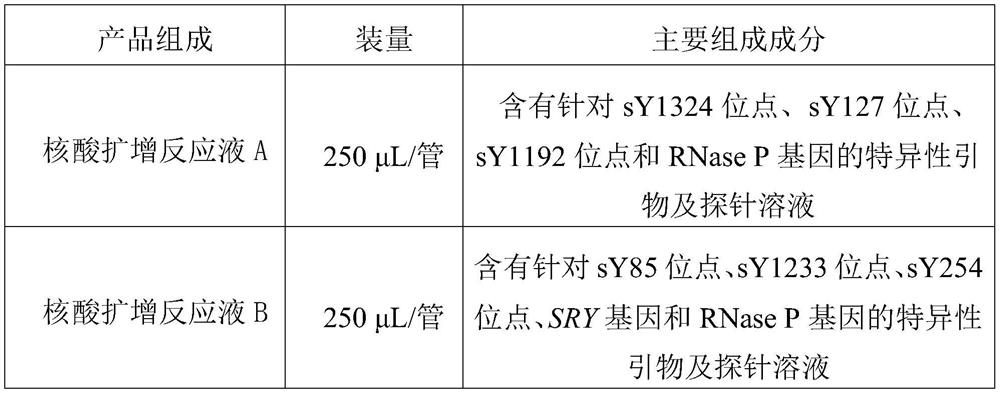
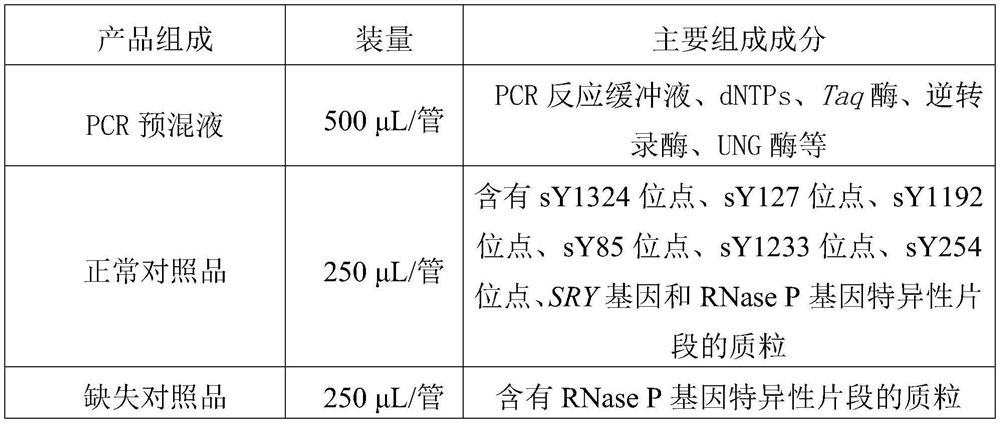
The invention relates to the field of molecular biology, and provides composition for detecting Y chromosome microdeletion. The composition comprises primers and probes with sequences as shown in SEQID NO: 1 to SEQ ID NO: 24. The invention also provides a product comprising the composition and a method using the composition. The composition has the characteristics of simple operation, short time,less pollution, accurate result and the like.
Owner:SHANDONG SHANDA HOSPITAL FOR REPRODIVE MEDICINE
Method of surface plasmon resonance (SPR) to detect genomic disorders for postnatal diagnosis
InactiveUS20100047789A1Detect directlySimultaneous measurementBioreactor/fermenter combinationsBiological substance pretreatmentsDiseaseSubtelomere
The present invention discloses using SPR technology to postnatally detect specific DNA loss or gain related to some genomic disorders. An efficient formula to make a mixed SAM that can greatly enhance the immobilization ability of the metal surface in SPR based techniques, which is good for the immobilization of DNA markers used for the identification of subtelomere imbalances and chromosome microdeletion syndromes is also disclosed.
Owner:CMED TECH
Amplification composition for Y chromosome microdeletion detection and kit thereof
ActiveCN109957616AReduce testing costsShort detection timeMicrobiological testing/measurementBiologyMultiplex pcrs
The invention discloses an amplification composition for Y chromosome microdeletion detection and a kit thereof. By using primers for amplifying 6 STS sites (AZFa: sY84, sY86; AZFb: sY127, sY134; AZFc: sY254, sY255) and 2 control sites (ZFX / ZFY, SRY), and the six STS sites closely related to the Y chromosome microdeletion are detected by a multiplex PCR combined agarose gel electrophoresis technology to determine the Y chromosome microdeletion. With the amplification composition of the present invention, six STS sites can be simultaneously detected by only two tube amplification reactions.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Method of surface plasmon resonance (SPR) technology to detect genomic disorders for prenatal diagnosis
InactiveUS20100279422A1Microbiological testing/measurementPolarisation-affecting propertiesDiseasePrenatal diagnosis
The present invention discloses using SPR technology to prenatally detect specific DNA loss or gain related to some genomic disorders. An efficient formula to make a mixed SAM that can greatly enhance the immobilization ability of the metal surface in SPR based techniques, which is good for the immobilization of DNA markers used for the identification of chromosome numerical abnormalities (such as chromosomes 13, 18, 21, X and Y related anomalies) and chromosome microdeletion syndromes (such as DiGeorge syndrome, etc) is also disclosed.
Owner:CMED TECH
Probe combination and kit for detecting microdeletion and microrepetition of Y chromosome and application of probe combination and kit
PendingCN114015787AConsistent target specificitySame hybridization conditionMicrobiological testing/measurementDNA/RNA fragmentationY chromosome microdeletionChromosome microdeletion
The invention relates to the field of chromosome variation detection, in particular to a probe combination and a kit for detecting microdeletion and microrepetition of a Y chromosome and application of the probe combination and the kit, and the probe combination comprises specific probes aiming at 25 detection points (6 basic points and 19 extension points) of the Y chromosome. When the probe combination provided by the invention is adopted for detection, only Y chromosome microdeletion and microrepetition, 25 point locations, variation interval range judgment and related gene judgment are needed to be simultaneously detected aiming at DNA to be detected in the same reaction tube, and a qualitative and quantitative method containing 6 basic point locations and 19 expansion point locations is adopted. The technical means can be applied to various specimens such as semen, peripheral blood, tissues and the like, and the sample size is required to be only 20-50ngDNA (Deoxyribose Nucleic Acid); the method is short in detection time, economical, simple and convenient, capable of reducing manual operation errors, suitable for various types of samples, visual and accurate in structure interpretation and easy to popularize.
Owner:王晴雪
A method and device for detecting microdeletion in chromosome sts region
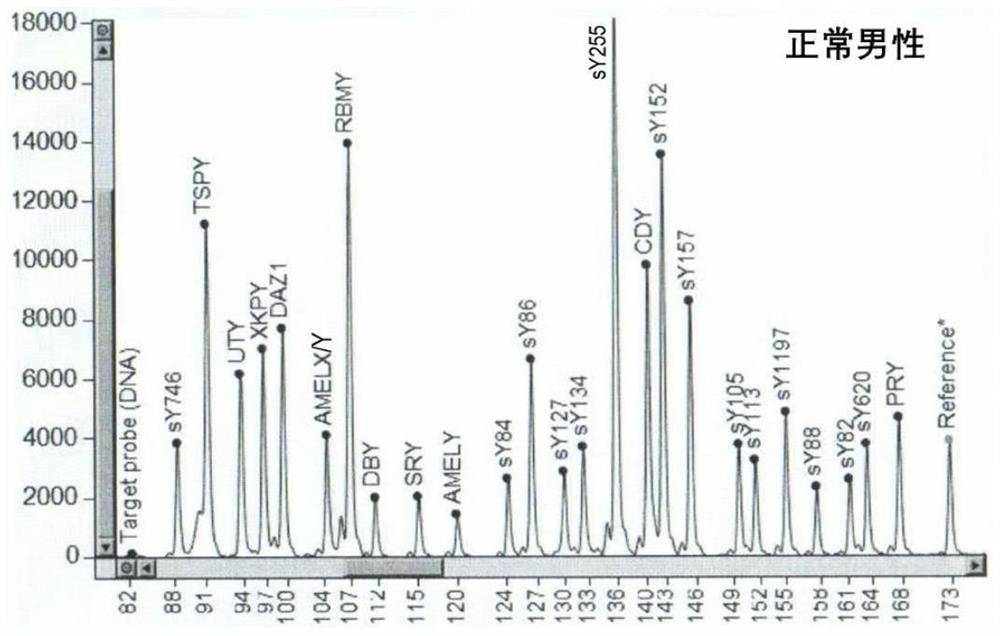
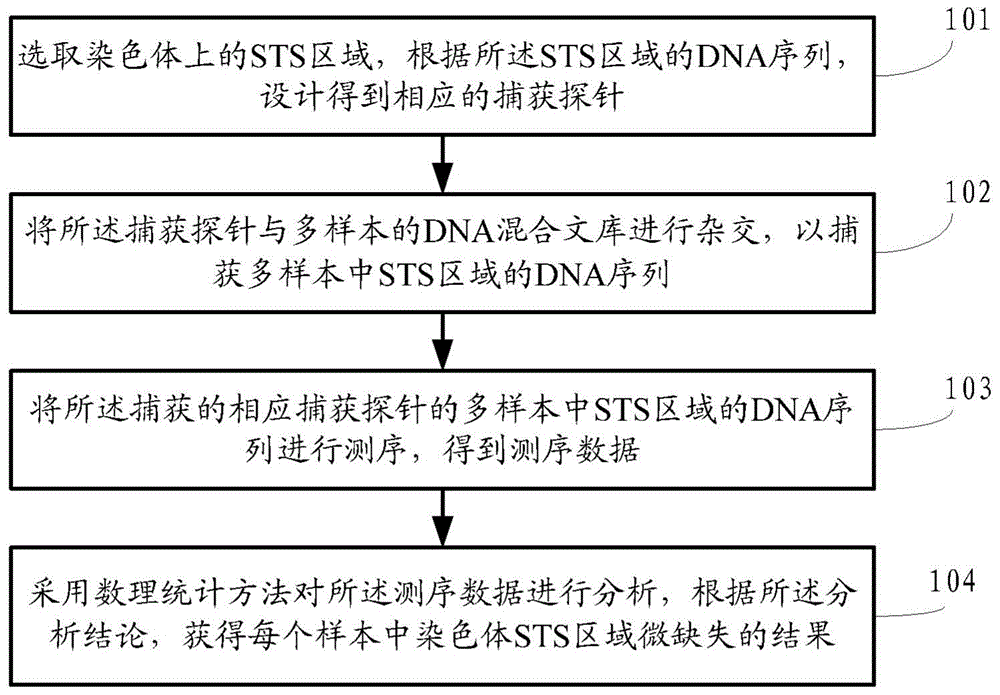
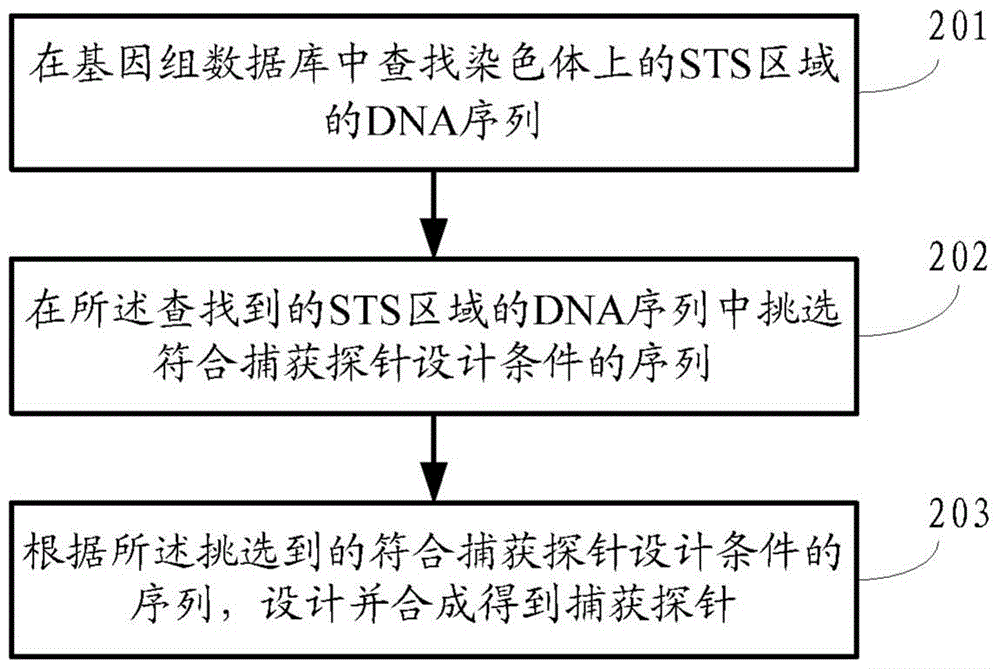
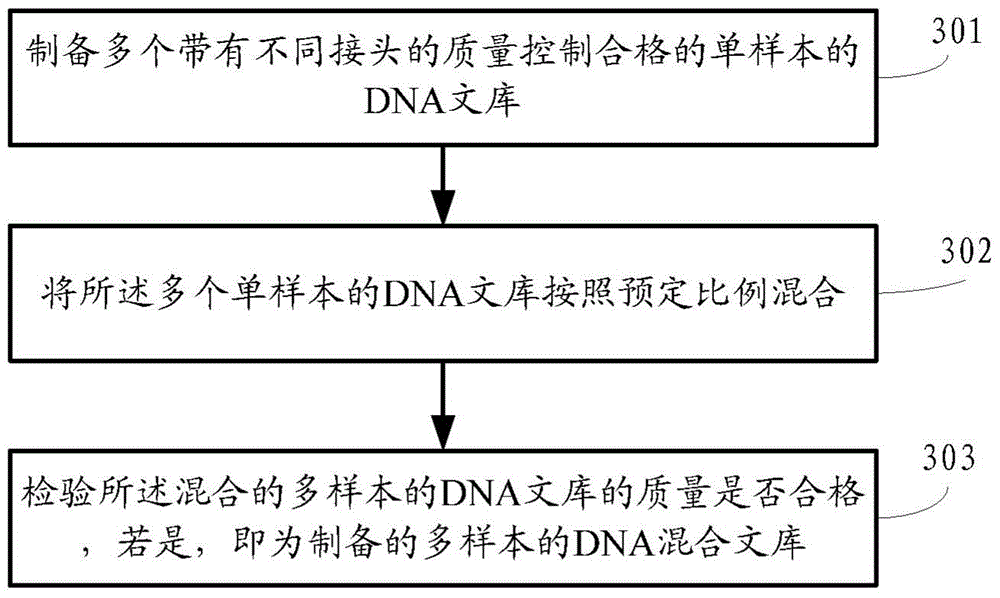
ActiveCN104145028BHigh sensitivityEfficient analysisMicrobiological testing/measurementA-DNAChromosome microdeletion
A method and a device for detecting microdeletion in a chromosome sequence tagged site (STS) area. The method comprises: selecting an STS area on chromosome, and designing according to a DNA sequence in the STS area to obtain a corresponding capture probe; hybridizing the capture probe and a multi-sample DNA hybrid library, so as to capture the DNA sequence in the STS area in the multi-sample; sequencing the captured DNA sequence in the STS area in the multi-sample of the corresponding capture probe, and obtaining sequencing data; analyzing the sequencing data through a mathematical statistics method, and obtaining a result regarding microdeletion in the chromosome STS area of each sample according to the analysis conclusion.
Owner:BGI GENOMICS CO LTD
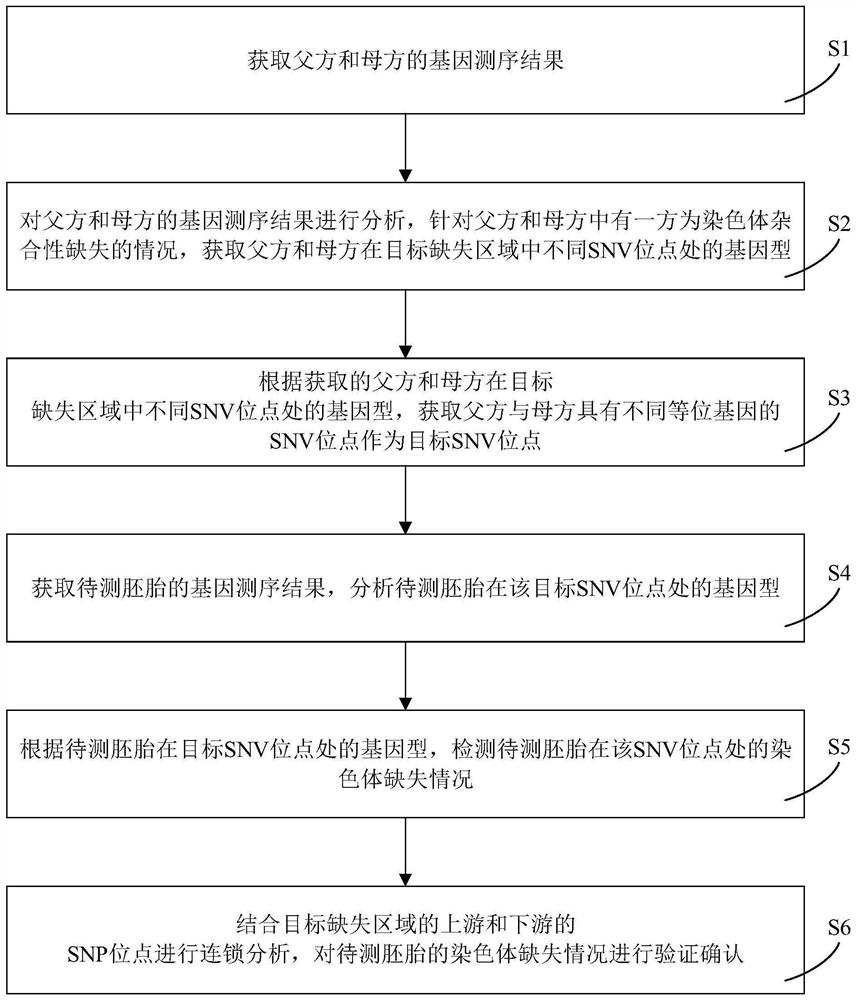
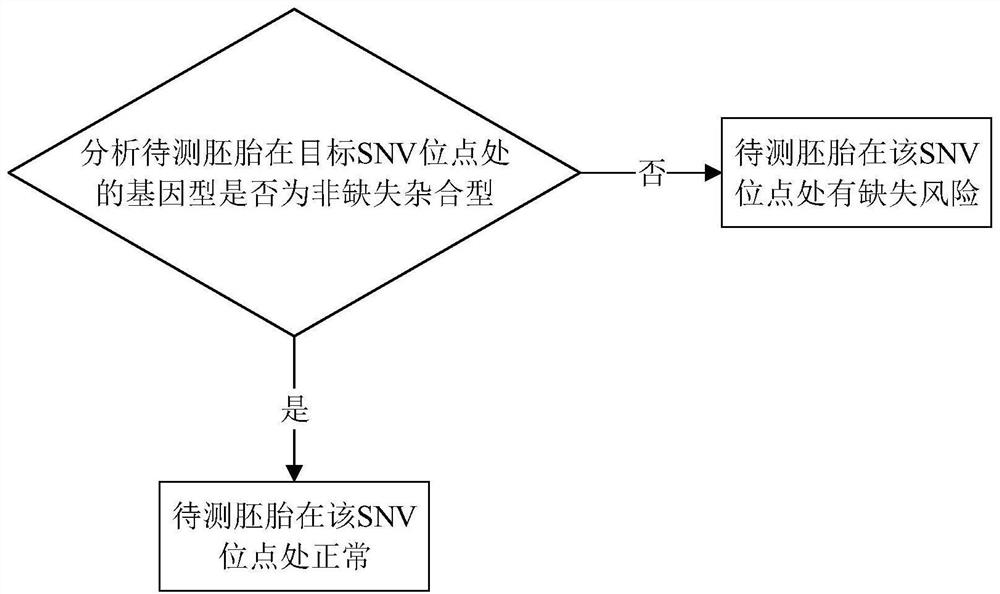
Embryo chromosome microdeletion detection method, device and equipment and storage medium
PendingCN113981070AImprove accuracyEasy to operateMicrobiological testing/measurementSequence analysisMedicineGenotype
The invention relates to an embryo chromosome microdeletion detection method, device and equipment and a storage medium. According to the embryo chromosome microdeletion detection method, the genotype of the SNV site in the chromosome microdeletion region of the parent party and the parent party is analyzed, the SNV site capable of being used for typing is found, then the genotype of the SNV site is detected in the embryo to be detected, and the chromosome type of the embryo to be detected is analyzed according to the genotype of the SNV site. According to the detection method, the accuracy of analyzing whether the embryo chromosome is microdeletion or not is high, breakpoint detection does not need to be performed on the embryo, the chromosome type of the embryo can be analyzed only according to the genotype of the SNV site, and the operation is simple.
Owner:SUZHOU BASECARE MEDICAL DEVICE CO LTD
A kit for detecting microdeletion of human y chromosome
ActiveCN104232779BMonitor stabilityReduce workloadMicrobiological testing/measurementFluorescenceChromosome microdeletion
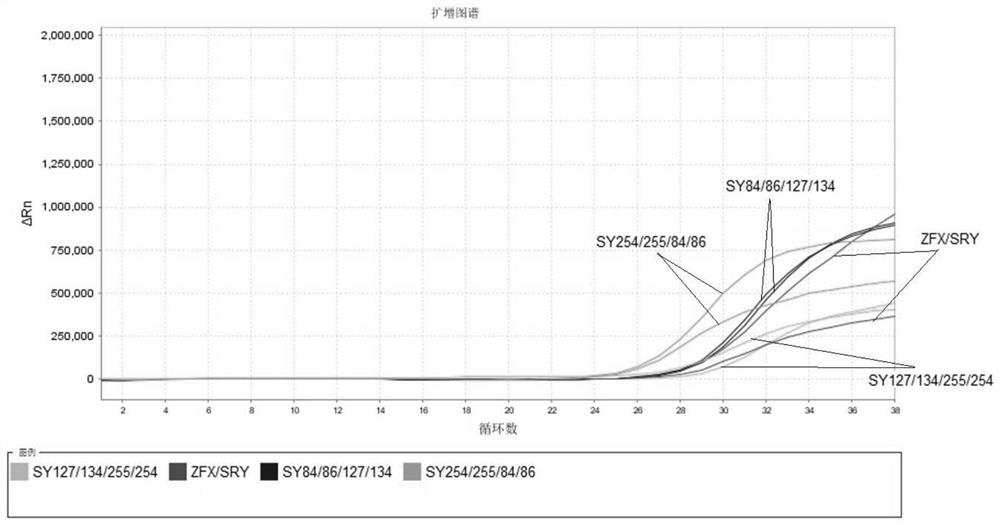
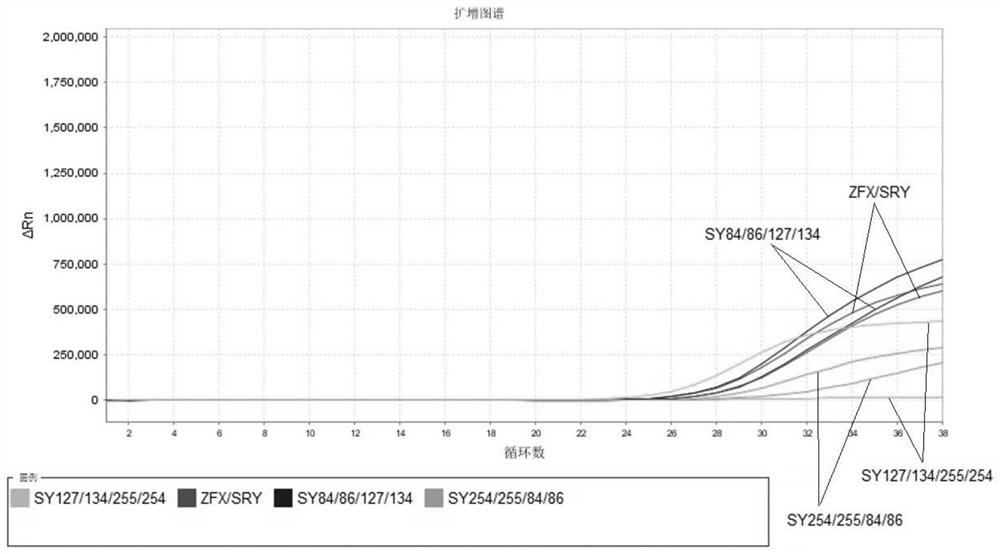
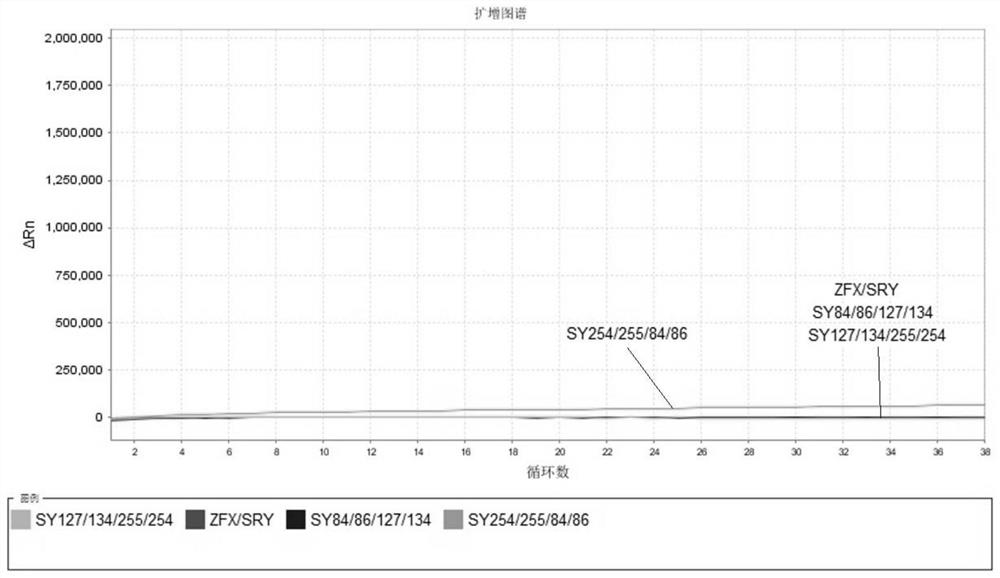
The invention relates to the field of chromosome deletion, in particular to a kit for detecting the microdeletion of a human Y chromosome. The kit comprises PCR (Polymerase Chain Reaction) reaction liquid I, PCR reaction liquid II and PCR reaction liquid III, wherein the PCR reaction liquid I comprises primers and fluorescent probes of specific amplification ZFX / Y, sY254, sY134 and SRY (Sex-determine Region of Y Chromosome) sites; the PCR reaction liquid II comprises primers and fluorescent probes of specific amplification ZFX / Y, sY84 and sY127 sites; the PCR reaction liquid III comprises primers and fluorescent probes of specific amplification ZFX / Y, sY255 and sY86 sites. According to the kit disclosed by the invention, by designing the specific fluorescent probes of different STS (Sequence Tagged Site) and three-pipe reaction systems are optimally combined, so that microdeletion types of the Y chromosome can be detected by one-time test; effective monitoring for different reaction systems is realized by the ZFX / Y.
Owner:亚能生物技术(深圳)有限公司
Equipment, kits and analysis systems for simultaneous prenatal screening of chromosomal and single gene diseases
Owner:BEIJING BIOBIGGEN TECH CO LTD +1
A genetic detection method for y-chromosome microdeletion
InactiveCN101575647BMeet the experimental conditionsSimple adjustment of primer concentrationMicrobiological testing/measurementClinical trialGenomic DNA
The invention discloses a gene detection method for Y chromosome microdeletion. After the genomic DNA of human peripheral blood is extracted, multiplex PCR primers are designed. The multiplex PCR reaction system includes N pairs of chimeric primer pairs that specifically amplify the STSs site in the AZF segment of Y chromosome and 1 pair of universal primer pairs. With the participation of N+1 pairs of primers, the multiplex PCR reaction amplifies the target DNA template through denaturation, annealing, and extension cycles in the same reaction system; take 10ul of PCR products, stain with EB, and undergo 3% agarose electrophoresis. The electrophoresis voltage was 4V / cm, and the electrophoresis time was 20 minutes; then the electrophoresis pattern was observed under the ultraviolet transilluminator to make a judgment on the result. Therefore, a detection method with simplified PCR reaction conditions, high amplification efficiency and easy clinical experiment operation is established.
Owner:成都军区昆明总医院
Azoospermia chromosome variation detection kit
PendingCN113981067AImprove detection efficiencyReduce testing costsMicrobiological testing/measurementDNA/RNA fragmentationMultiplexY chromosome microdeletion
The invention belongs to the technical field of biological detection, and discloses an azoospermia chromosome variation detection kit. According to the invention, on the basis of retrospective analysis of detection results of chromosome karyotype and Y chromosome microdeletion of patients suffering from azoospermia and establishment of an azoospermia genetic database in China, a multiplex fluorescent quantitative PCR method is adopted to establish a primer probe and a kit for realizing azoospermia chromosome variation detection at one time, the clinical male infertility reason detection efficiency is improved, the cost is reduced, and the period is shortened; meanwhile, the research specially aims at Chinese populations, the clinical actual requirements of China are better met, and the positive detection rate is increased. The cause of male infertility is determined from the molecular level, so that unnecessary treatment is avoided, and the problem that the clinical mutation deletion rate of male offspring is increased due to the application of ICSC is reduced. In addition, the most advanced drying technology is adopted, the detection sensitivity can be improved, the clinical detection process is simplified, and actual use and popularization are more convenient.
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV +1
Amplification composite for detecting microdeletion of Y-chromosome and detection kit
ActiveCN102912028BReliable resultsThe test results are credibleMicrobiological testing/measurementDNA/RNA fragmentationGeneticsFluorescent pcr
The invention relates to amplification composite for detecting the microdeletion of Y-chromosome and a detection kit, belonging to the field of biotechnical detection. The amplification composite for detecting the microdeletion of Y-chromosome can be amplified to as many as 30 sites related to the microdeletion detection of the Y-chromosome through one reaction. The detection kit detects the microdeletion of the Y-chromosome through the quantitative fluorescent PCR (Polymerase Chain Reaction) method by using the amplification composite. The microdeletion abnormality of the Y-chromosome is determined according to the existence of an amplification product and the quantity of the amplification product. A large quantity of sites enables the detection result to be more convincible and can provide much more and more detailed information for determining the deletion type, and the quantitative detection of partial deletion and repetition can be realized. The detection kit is easier and more convenient to operate, only one PCR amplification and one sequencer detection reaction are needed to complete the detection of one sample, the whole process needs 4-5 h, and the operation intensity and the detection time are greatly reduced.
Owner:BEIJING MICROREAD GENE TECH
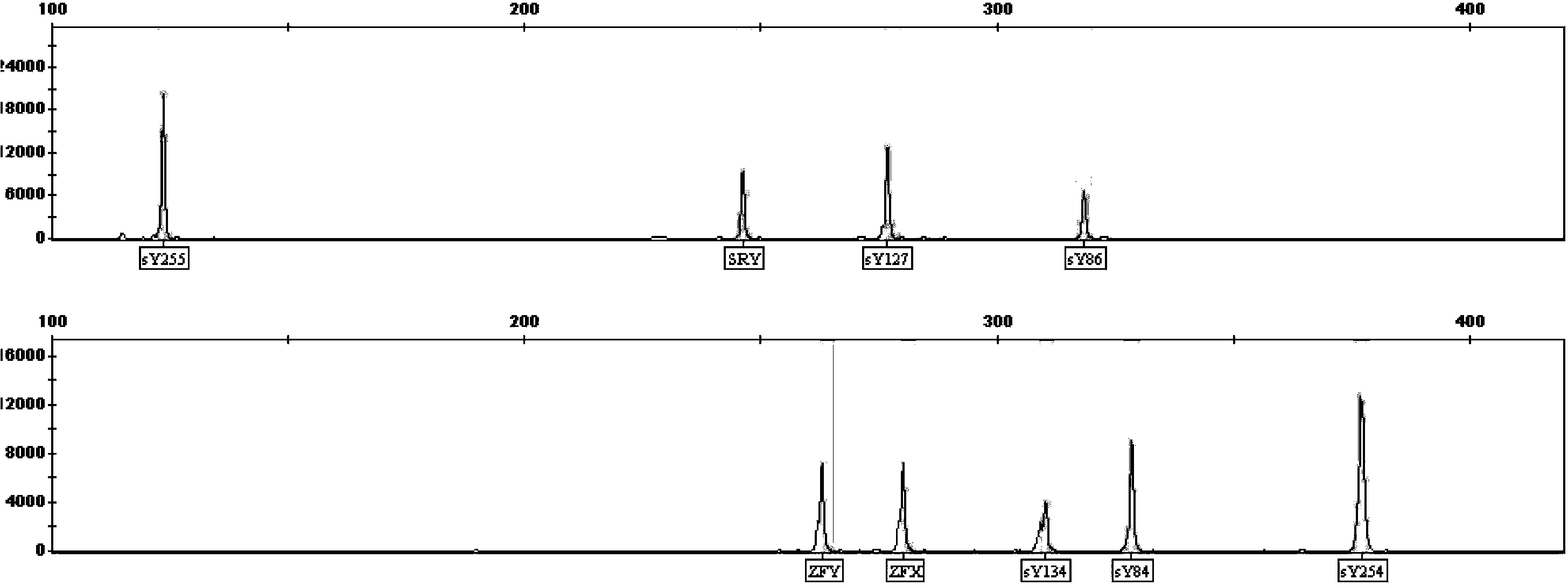
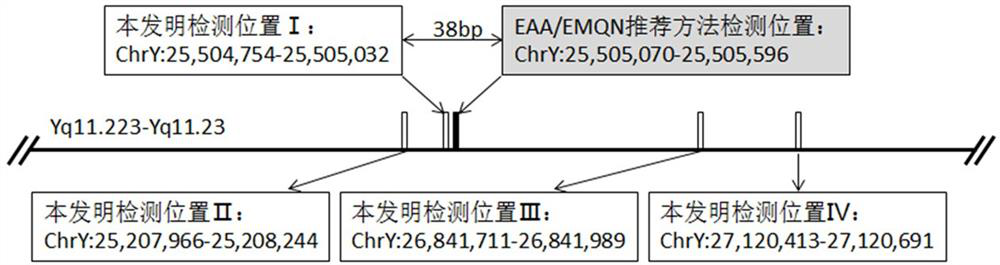
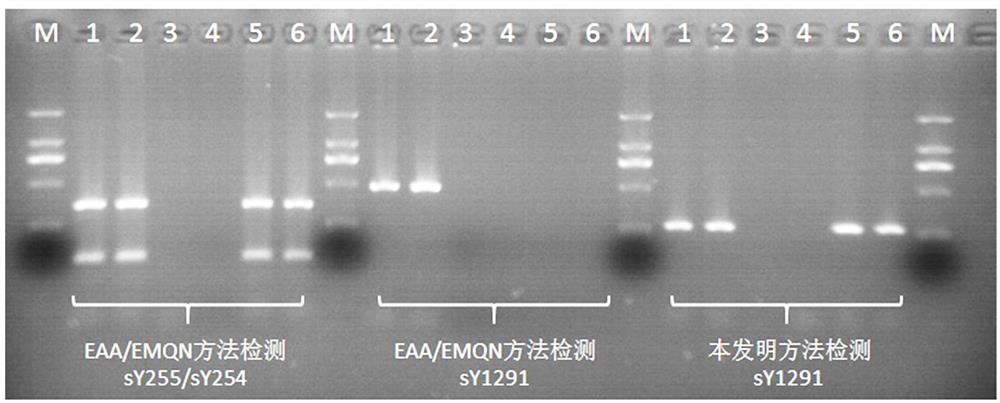
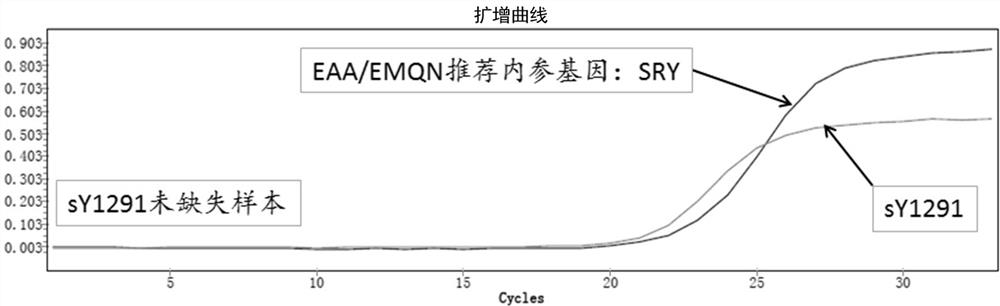
A detection method and application of human y chromosome label site sy1291
ActiveCN107502668BExpand the scope of detectionOvercoming the problem of inconsistent test resultsMicrobiological testing/measurementDNA/RNA fragmentationFluoProbesElectrophoreses
The invention belongs to the technical field of in vitro nucleic acid detection, and specifically relates to a detection method of a human Y chromosome tag site sY1291 and an application thereof. The present invention provides a new sY1291 site detection strategy, that is, redesign specific PCR amplification primers in the 38bp large fragment repeat region upstream of the sY1291 site detection region recommended by EAA / EMQN, and the new PCR amplification primers detect region I The 5' end of the site is 38 bp away from the 3' end of the sY1291 site detection region recommended by EAA / EMQN, and the new PCR amplification primers can simultaneously detect four regions I, II, III, and IV. The detection strategy of the present invention combines agarose electrophoresis, fluorescent label primer capillary electrophoresis or fluorescent probe method to form different detection methods of sY1291 site. The method for detecting the tag site sY1291 of the human Y chromosome provided by the present invention has strong specificity and a wide detection area of the Y chromosome, and is more suitable for the expanded detection of the AZF microdeletion of the human Y chromosome.
Owner:上海五色石医学科技有限公司
New chromosomal microdeletion/microduplication syndrome detection system and kit
ActiveCN104651516BMultiple diseases involvedMultiple coverage precisionMicrobiological testing/measurementMultiplexDECIPHER
The invention discloses a novel chromosome microdeletion / microduplication syndrome detection system which mainly comprises 643 pairs of probes, multiplex PCR (polymerase chain reaction) amplification primers and correlation detection reaction reagents. The detection process comprises the following steps: hybridizing a probe set with target nucleic acid in the sample; connecting the hybridized probe; amplifying the connecting probe; and detecting the amplification product to determine the existence or quantity of the target nucleic acid in the sample. The platform can implement systematic screening and detection on 24 chromosome aneuploids, 24 chromosome telomere abnormities, more than 70 chromosome microdeletion / microduplication syndromes and multiple monogenic disease mutation hot spots at one time by using the optimized probe set and reaction system. Compared with the existing like detection techniques (such as MLPA and the like), the detection system covers nearly all definite pathogenic regions with abnormal number of copies in the DECIPHER database, has the advantages of wide detection range, high detection precision and low cost, and is simple to operate.
Owner:SUZHOU MUNICIPAL HOSPITAL +1
A product for detecting chromosome 16p11.2 microdeletion
ActiveCN105624308BImprove accuracyEasy to operateMicrobiological testing/measurementPhysiologyGenetics
Owner:PEKING UNION MEDICAL COLLEGE HOSPITAL CHINESE ACAD OF MEDICAL SCI
Primer combination and kit for y chromosome microdeletion detection
ActiveCN106591442BImprove economySimple and fast operationMicrobiological testing/measurementDNA preparationMultiplexElectrophoreses
The invention relates to a primer combination and kit for chromosome microdeletion. By the use of a multiple PCR combined with agarose gel electrophoresis technology, seven STS loci closely associated with the Y chromosome microdeletion are detected to judge the microdeletion conditioof a Y chromosome. Seven STS loci with high specificity and sensitivity (sY84, sY86, sY127, sY134, sY254, sY255 and sY160) are chosen as detection loci, at the same time two loci ZFX / Y and SRY are utilized as quality control loci, a primer is designed aiming at each locus respectively, and products of the nine loci after multiplex amplification are directly put on the agarose gel to be conducted electrophoresis detection. By the use of the primer combination and detection system, the Y chromosome microdeletion can be detected only needing single tube amplification reaction.
Owner:北京圣谷智汇医学检验所有限公司
A composition of primers and probes for detecting y-chromosomal microdeletion, a detection method and a kit for non-diagnostic purposes
ActiveCN113136418BImprove the detection rateLow costMicrobiological testing/measurementDNA/RNA fragmentationY chromosome microdeletionPcr method
Owner:BEIJING CHAOYANG HOSPITAL CAPITAL MEDICAL UNIV +1
A method for detecting chromosomal microdeletions and microduplications in human embryos
ActiveCN104745718BHigh sensitivityLow costMicrobiological testing/measurementEmbryoChromosome microdeletion
Owner:BEIJING ZHONGYI KANGWEI MEDICAL INSTR
Multiplex PCR primer sets, kits and applications for detection of Y chromosome microdeletions
ActiveCN107012234BLow costEasy to operateMicrobiological testing/measurementDNA/RNA fragmentationMultiplexBase J
The invention relates to the field of gene deletion detecting, in particular to a multiplex PCR (polymerase chain reaction) primer set for detection Y chromosome microdeletion, kit and application. The multiplex PCR primer set comprises a universal primer and a specific primer, the universal primer has base sequences shown as SEQ ID NO:1 and SEQ ID NO:2, a 5' terminal of the base sequence shown as the SEQ ID NO:1 is marked with a fluorophore, and the specific primer comprises a primer having base sequences shown as SEQ ID NO:3-SEQ ID NO:22. The defect that conventional multiplex PCR is low in sensitivity and non-specific amplification is overcome; through a method combining a novel stable universal primer-multiplex PCR reaction system with a fluorescent labeling universal primer, an amplification product is subjected to capillary electrophoresis analysis, so that high sensitivity of detection is guaranteed, cost is effectively lowered, and large-sample-size efficient detection can be realized.
Owner:THE FIRST AFFILIATED HOSPITAL OF ZHENGZHOU UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com