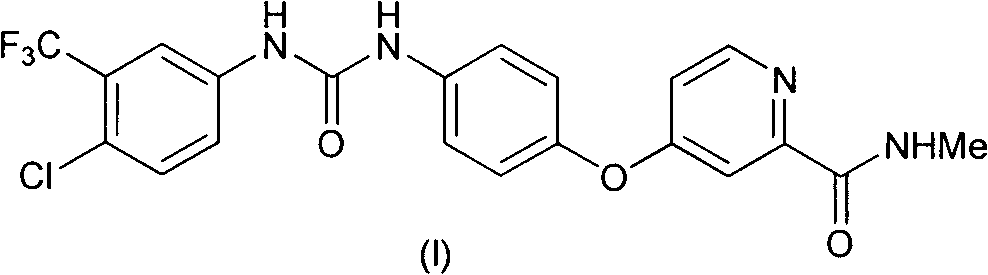
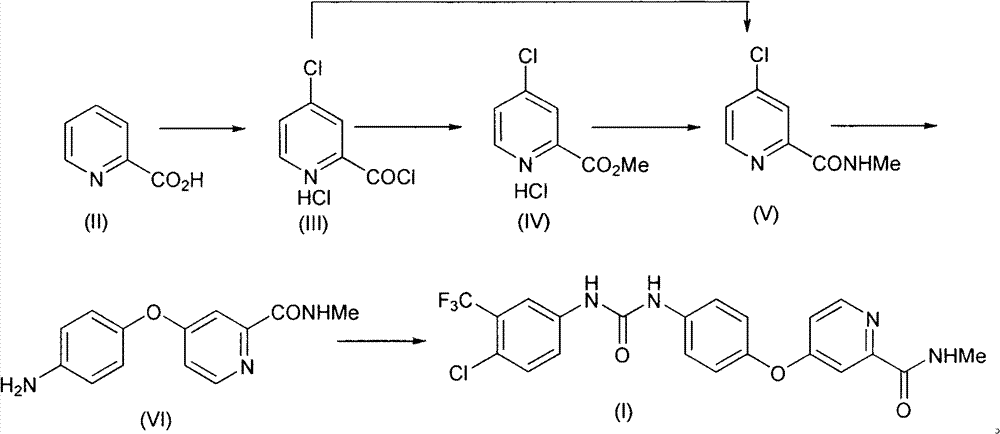
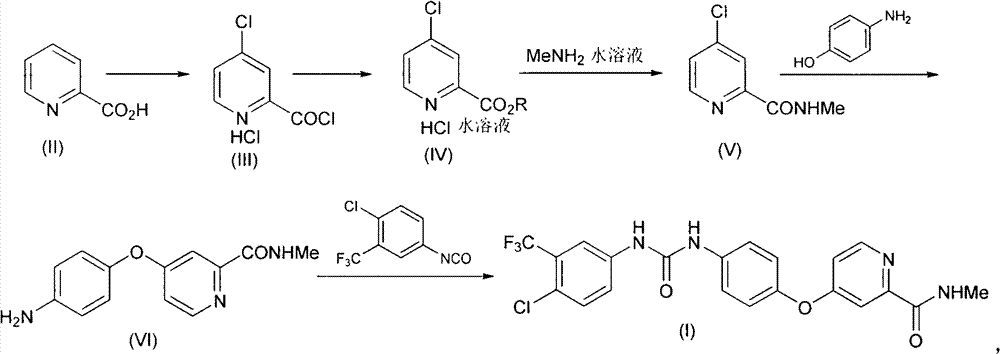
Method for preparing sorafenib
An amino and compound technology, applied in the field of preparing sorafenib, can solve the problems of cumbersome operation, low yield, increased cost and the like, and achieve the effects of simplified operation, simple operation and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Synthesis of embodiment one 4-chloropyridine-2-formyl chloride hydrochloride (III)
[0037] Add 2.7L of thionyl chloride and 90mL of N,N-dimethylformamide into the reaction flask, and stir thoroughly; then add 1.5Kg of pyridine-2-carboxylic acid, raise the temperature to 75°C, react for 24 hours, and distill under reduced pressure to fully remove Thionyl chloride was used to obtain the crude product of 4-chloropyridine-2-formyl chloride hydrochloride (III), which was directly carried out to the next reaction.
Embodiment 2
[0038] The synthesis of embodiment two 4-chloropyridine-2-formic acid methyl ester hydrochloride (IV)
[0039] Add 1L of toluene to the above crude compound (III), heat to 50°C, and add 1L of methanol under full stirring. After the dropwise addition, filter to obtain a solid with a purity of 70%; add 7L of water and 3L of ethyl acetate to the solid , liquid separation to obtain the aqueous solution of compound (IV), directly used in the next step reaction. The purity of compound (IV) in aqueous solution is 97%.
Embodiment 3
[0040] The synthesis of embodiment three 4-chloro-N-methylpyridine-2-carboxamides (V)
[0041] The above aqueous solution of compound (IV) and 2.3 Kg of 30% methylamine aqueous solution were thoroughly mixed and then stirred at room temperature for 2 hours. Subsequently, the reaction solution was extracted three times with 5 L of ethyl acetate each time, and the organic phase was obtained by liquid separation. The organic phase was extracted once again with 5L saturated brine, and after the organic phase was dried, the organic solvent was removed under reduced pressure to obtain 1.77Kg 4-chloro-N-methylpyridine-2-carboxamide (V), which was directly used in the next step reaction, The three-step reaction yield is 85%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com