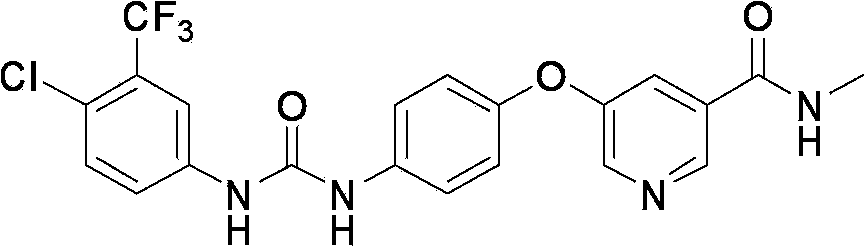
Crystal form A of Sorafenib and preparation method thereof
A fennel, crystal form technology, applied in the field of medicine and chemical industry, can solve the problems of long reaction cycle and unsuitability for industrial production, and achieve the effect of not easy to decompose, good stability and high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14-
[0040] Example 14- Preparation of [4-[3-(4-chloro-3-trifluoromethylphenyl)ureide]phenoxy]-pyridine-2-carboxamide (crude sorafenib)
[0041]
[0042] 52.3 g of 4-(4-aminophenoxy)-N-methylpyridine-2-carboxamide were suspended in 146 g of ethyl acetate, and the suspension was heated to about 40°C. Then 50 g of 4-chloro-3-trifluoromethylphenyl isocyanate dissolved in 53 g of ethyl acetate was added at a controlled rate to keep the reaction temperature below 60°C. Cool to 20°C over 1 hour, then the mixture is stirred for a further 30 minutes and the product is filtered off. Washed with 30 g of ethyl acetate, and then dried under vacuum at 50°C to obtain 93 g of sorafenib with a yield of 93% and a purity of 57.8% by HPLC. MS(m / z):[M-H]465.15
Embodiment 24-
[0043] Example 24- Preparation of crystalline form A of [4-[3-(4-chloro-3-trifluoromethyl-phenyl)ureide]phenoxy]-pyridine-2-carboxamide (Sorafenib)
[0044]1g of the obtained Sorafenib in Example 1 was added to a mixed solvent of 20ml of dichloromethane and 3.3ml of methanol, heated to reflux for 10min, stopped heating, and after naturally cooling to room temperature, continued stirring and crystallization for 2 hours, suction filtration, washing, Vacuum drying gave 0.92 g of off-white solid with a yield of 92%, HPLC: 99.7%.
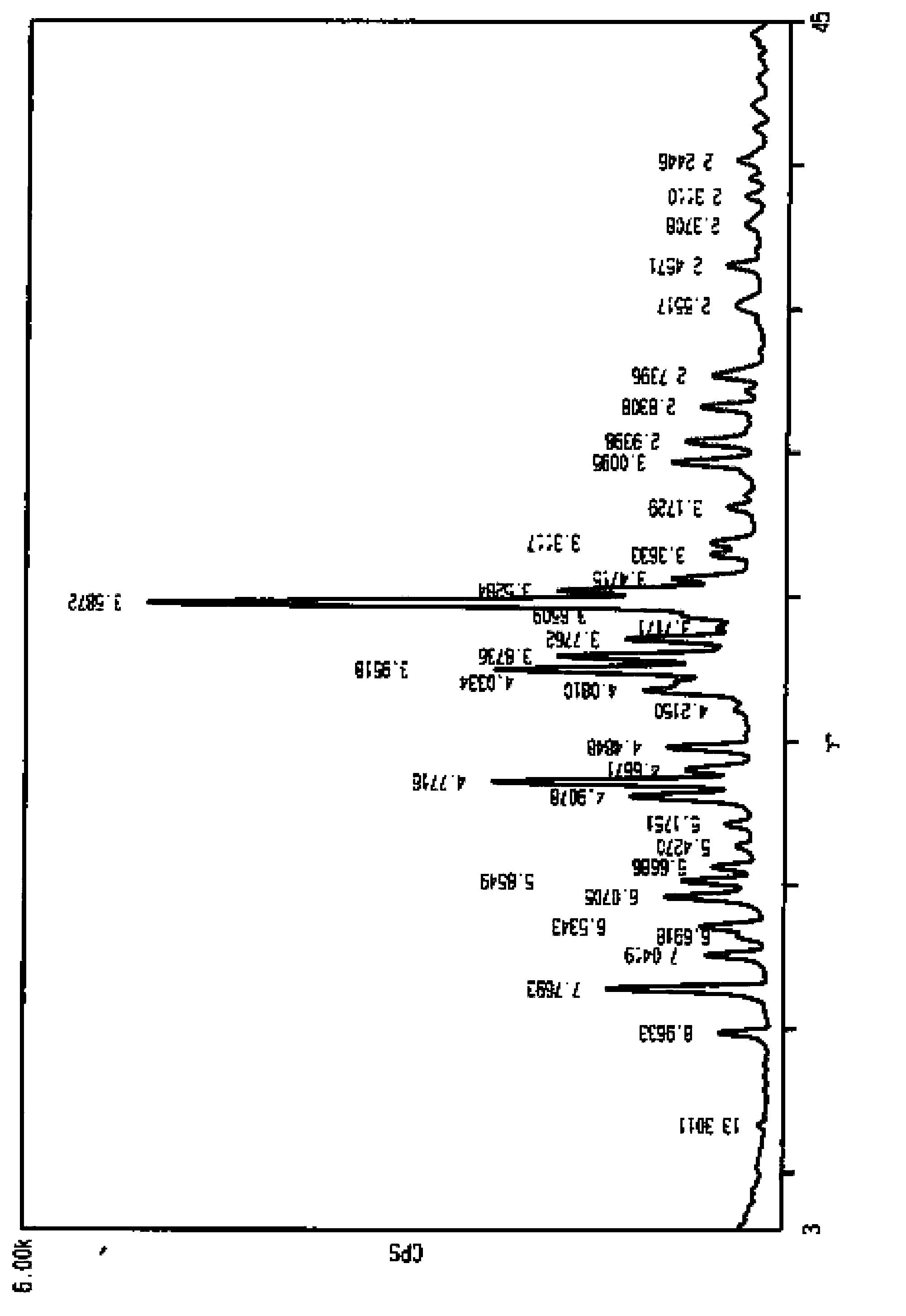
[0045] The obtained crystal form is determined by X-ray powder diffraction pattern, and its X-ray powder diffraction represented by 2θ angle is at 6.6±0.2°, 9.9°±0.2°, 11.4±0.2°, 12.6±0.2°, 13.2±0.2°, 13.5±0.2°, 14.6±0.2°, 15.1±0.2°, 15.6±0.2°, 18.1±0.2°, 18.6±0.2°, 19.8±0.2°, 21.8±0.2°, 22.5±0.2°, 22.9±0.2°, There are characteristic peaks at 23.5±0.2°, 24.8±0.2°, 25.2±0.2°, 29.7±0.2°, 30.4±0.2°, 31.6±0.2°, 32.7±0.2°, 36.5±0.2°.
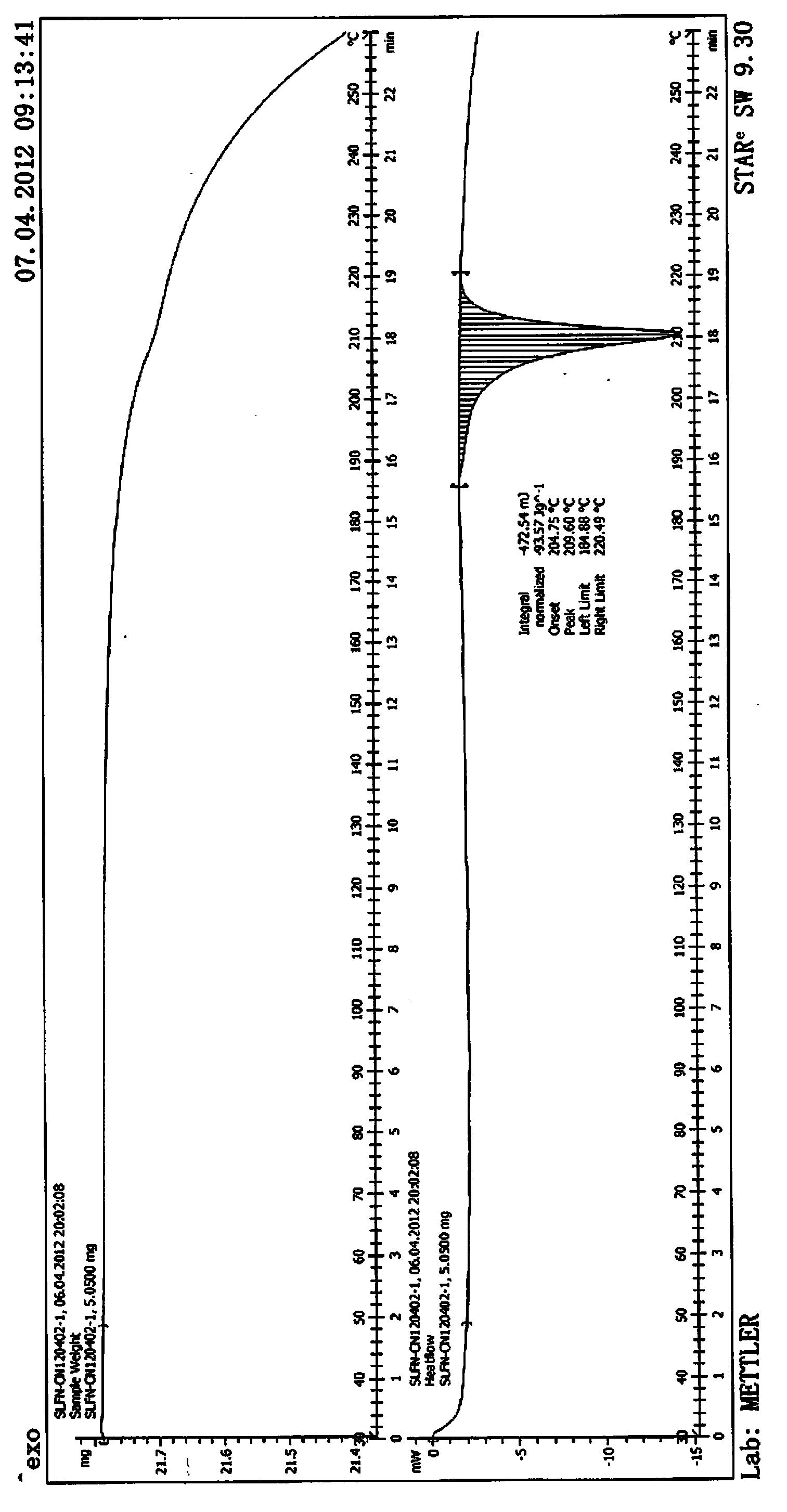
[0046] In differentia...
Embodiment 34-
[0047] Example 34-[4-[3-(4-Chloro-3-trifluoromethyl-phenyl)ureide]phenoxy]-pyridine-2-carboxamide (Sorafenib) Preparation of Form A
[0048] 1g of Sorafenib obtained in Example 1 was added to a mixed organic solvent of 10ml of dichloromethane and 15ml of ethanol, heated to reflux for 20min, cooled to -5°C, continued to stir and crystallize for 1 hour, washed with suction and dried to obtain 0.86 g off-white solid, yield 86%, HPLC: 99.2%.
[0049] According to the XRPD data, the obtained crystal form is the crystal form A of sorafenib described in the present invention.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com