Novel sorafenib tosylate polycrystalline form and preparation method thereof
A technology of p-toluenesulfonate and p-toluenesulfonic acid, which is applied in the field of sorafenib p-toluenesulfonate polymorph A and its preparation, can solve unfavorable industrial scale-up production, cumbersome operation of polymorph I, low Temperature and other issues, to achieve the effect of simple operation, high product yield and good repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Embodiment 1 Preparation of Sorafenib tosylate (I) polymorphic form A
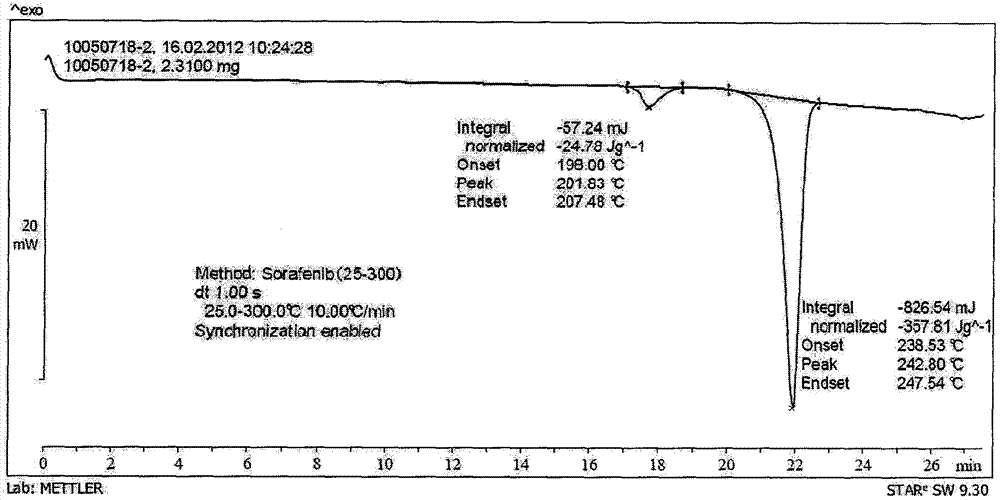
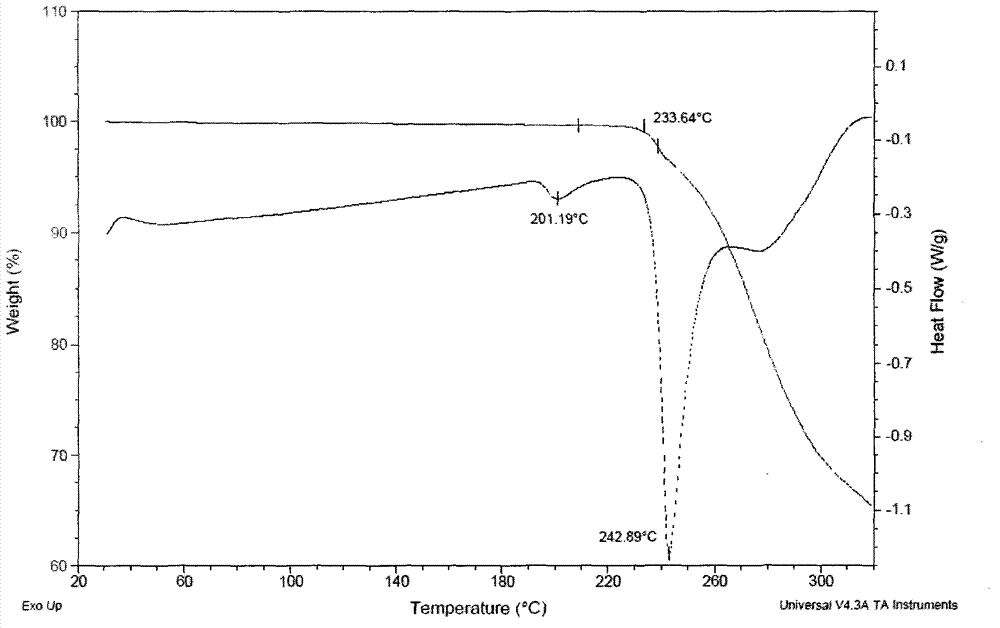
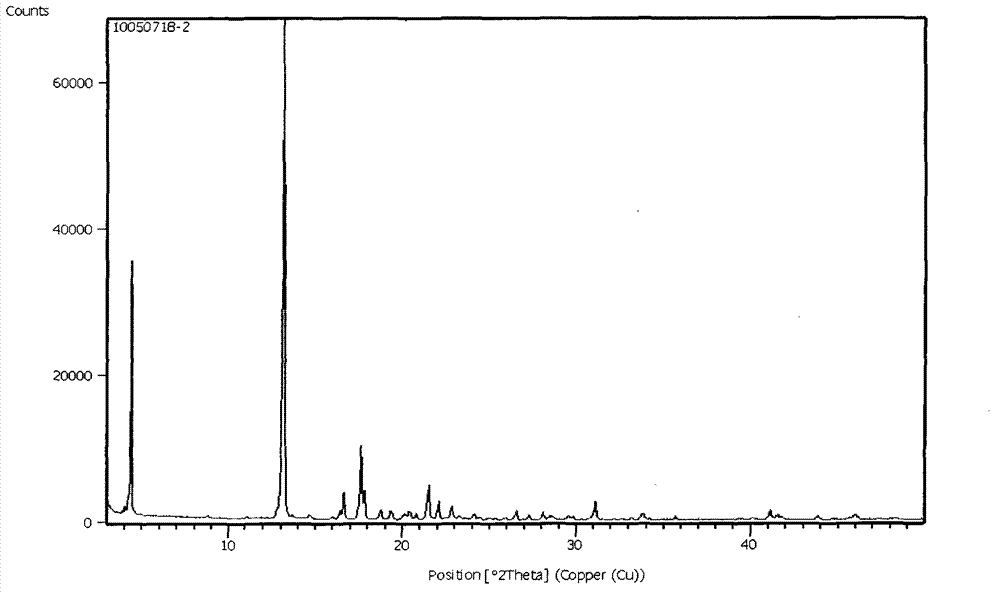
[0038] Add 300 g of sorafenib free base, 130 g of p-toluenesulfonic acid, 2.5 L of n-propanol, and 500 mL of water into the reaction flask. Heat the system to 80 degrees within 5 hours until the system is clear. Cool to 0° C., filter the precipitated crystals, and dry thoroughly to obtain 355 g of polymorphic form A of sorafenib p-toluenesulfonate (I), with a yield of 86% and a purity of 99.9%.
Embodiment 2
[0039] The preparation of embodiment two Sorafenib tosylate (I) polymorph A
[0040] Add 300 g of sorafenib free base, 130 g of p-toluenesulfonic acid, 2.5 L of isopropanol, and 500 mL of water into the reaction flask. Heat the system to 75 degrees within 4 hours until the system is clear. Cool to 10° C., filter the precipitated crystals, and fully dry to obtain 384 g of polymorphic form A of sorafenib p-toluenesulfonate (I), with a yield of 93% and a purity of 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com