Sorafenib antitumor platinum (II) complex targeted at human lung cancer drug-resistant cells and preparation method and application thereof
A drug-resistant cell and anti-tumor technology, which is applied in the fields of anti-tumor drugs, pharmaceutical formulations, platinum group organic compounds, etc., can solve the problems of undiscovered anti-tumor activity research, and achieve superior anti-tumor activity in vitro
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Weigh 1.0mmol ligand SFB and 1.0mmol cis-PtCl respectively 2 (DMSO) 2 , placed in a 100.0mL high-temperature pressure-resistant bottle, add 25.0mL of anhydrous methanol and 5.0mL of acetone solution, heat at 45°C for 28 hours, cool to room temperature, a large amount of yellow product precipitates, suction filter, yellow The product was washed successively with methanol, acetone and ether, and dried under vacuum at 45°C to finally obtain complex 1 with a yield of 98.0%.
[0027] Identification of the obtained complex 1:
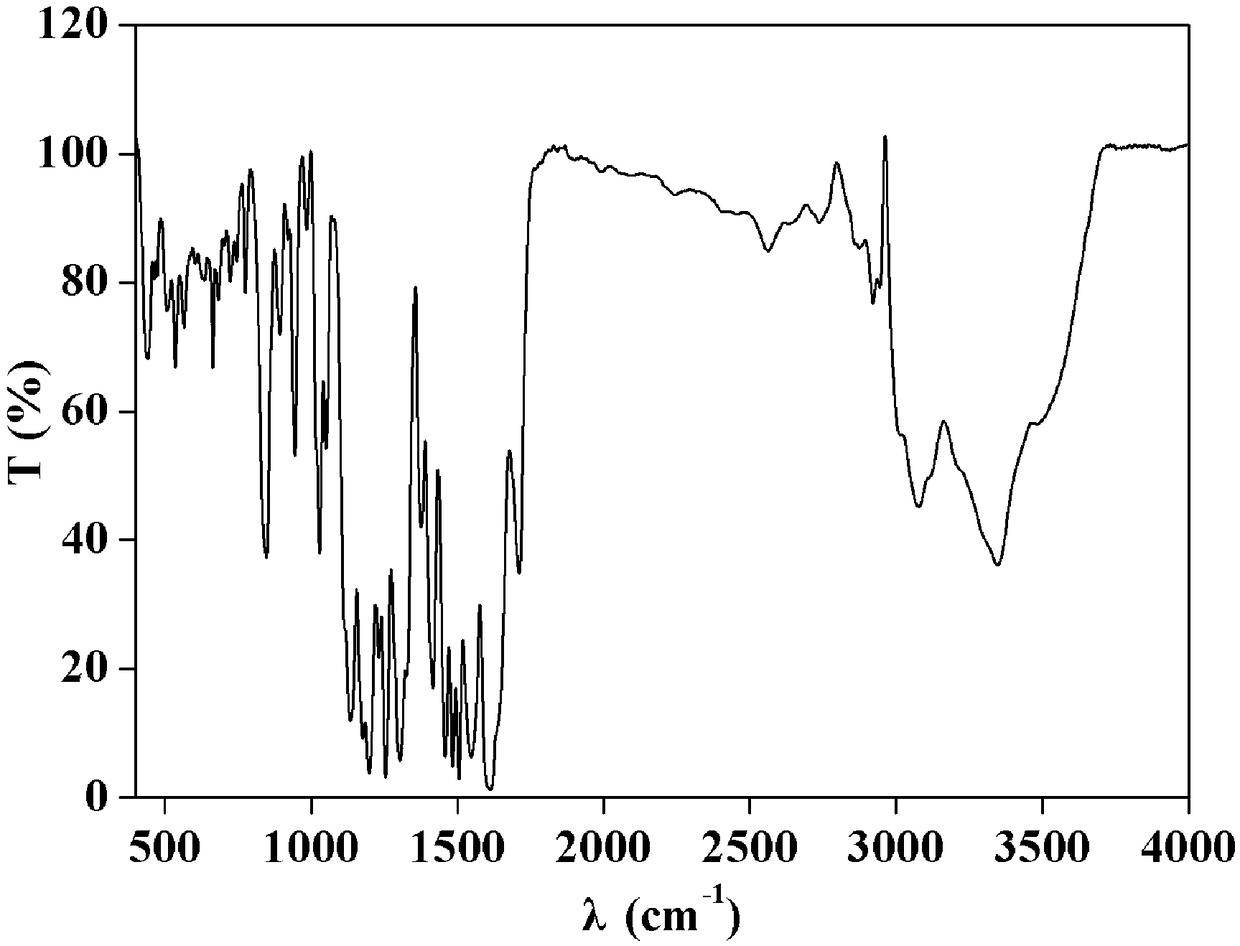
[0028] (1) Infrared spectrum, its spectrogram is as follows figure 1 shown.
[0029] IR(KBr):3348,3080,2920,2563,1711,1615,1547,1484,1418,1303,1254,1198,1176,1134,1050,1030,944,848,663,535,436cm -1 .
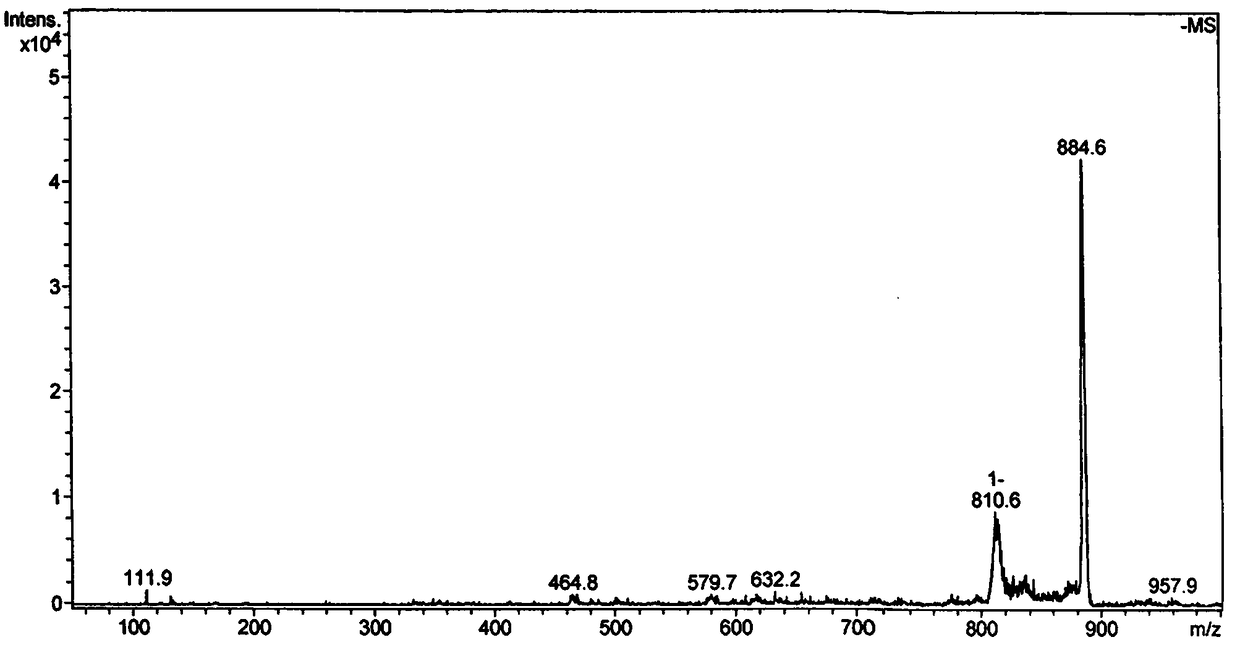
[0030] (2) Electrospray mass spectrometry, its spectrogram is as figure 2 shown.
[0031] ESI-MS m / z:884.6[M-H+DMSO] - , where M is the molecular weight of complex 1.
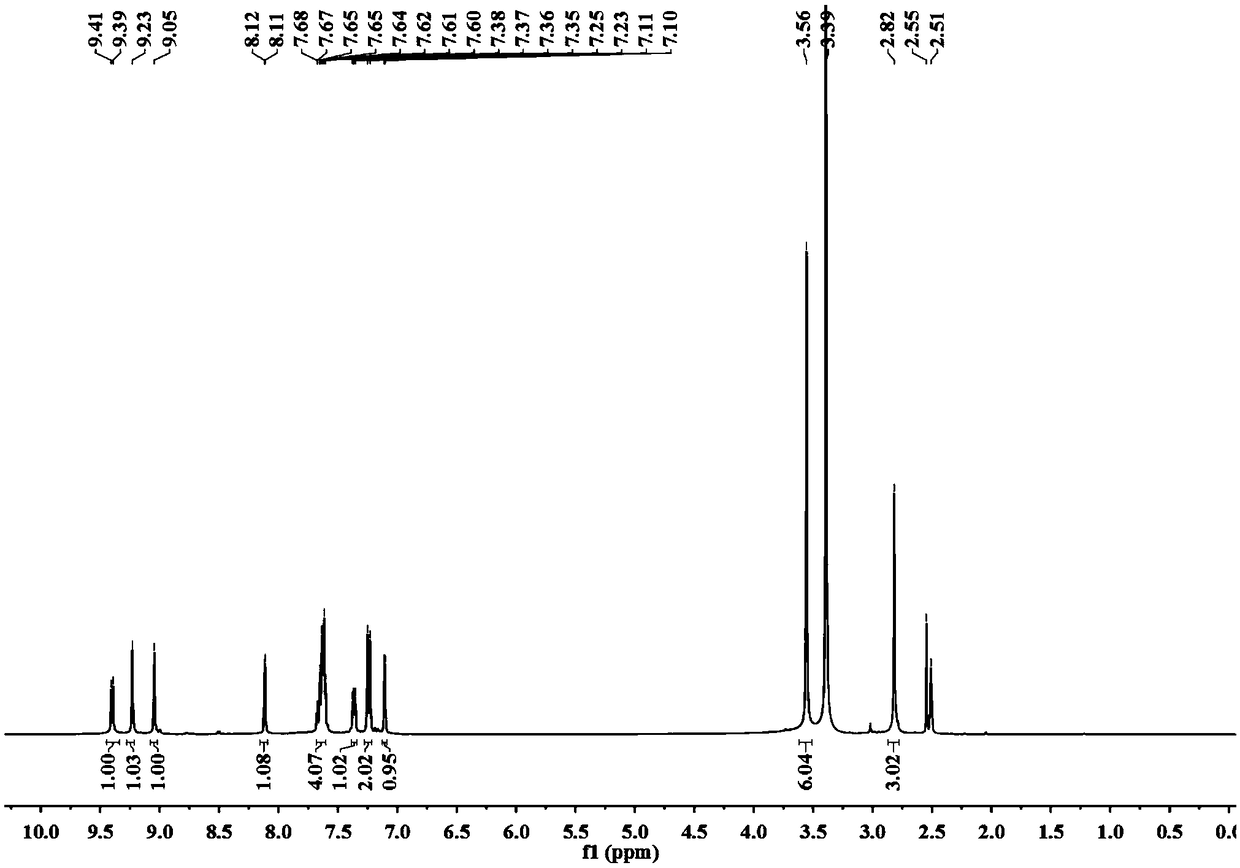
[0032] (3) Proton NMR spectrum, such as image 3 shown.
[0033] 1 H NMR...
Embodiment 2
[0042] Weigh the ligands 1.0mmol SFB and 5.0mmol cis-PtCl respectively 2 (DMSO) 2 , placed in a 100.0mL high-temperature and pressure-resistant bottle, add 50.0mL of absolute ethanol and 10.0mL of acetone solution, heat at 65°C for 36 hours, cool down to room temperature, a large amount of yellow product precipitates, and suction filter, The yellow product was washed successively with methanol, acetone and ether, and dried under vacuum at 45°C to finally obtain complex 1 with a yield of 85.5%.
Embodiment 3
[0044] Weigh 5.0mmol ligand SFB and 1.0mmol cis-PtCl respectively 2 (DMSO) 2 , placed in a 100.0mL high-temperature and pressure-resistant bottle, add 40.0mL of acetonitrile and 20.0mL of acetone solution, heat at 37°C for 12 hours, cool down to room temperature, a large amount of yellow product precipitates, suction filter, yellow product After washing with methanol, acetone and ether in sequence, and vacuum drying at 45°C, complex 1 was finally obtained with a yield of 75.0%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com