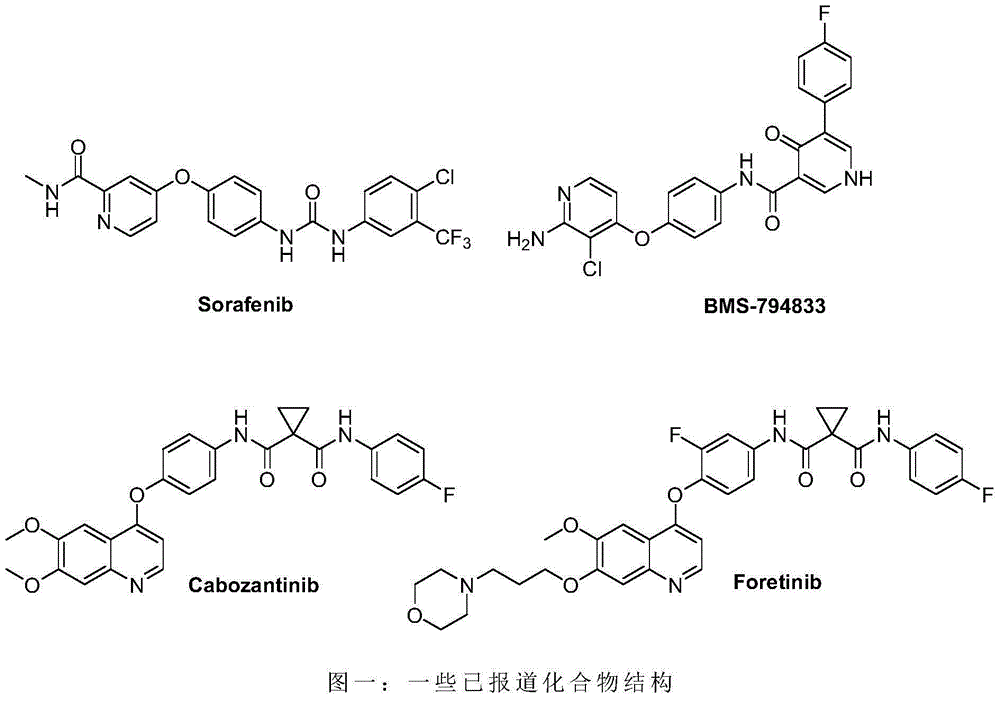
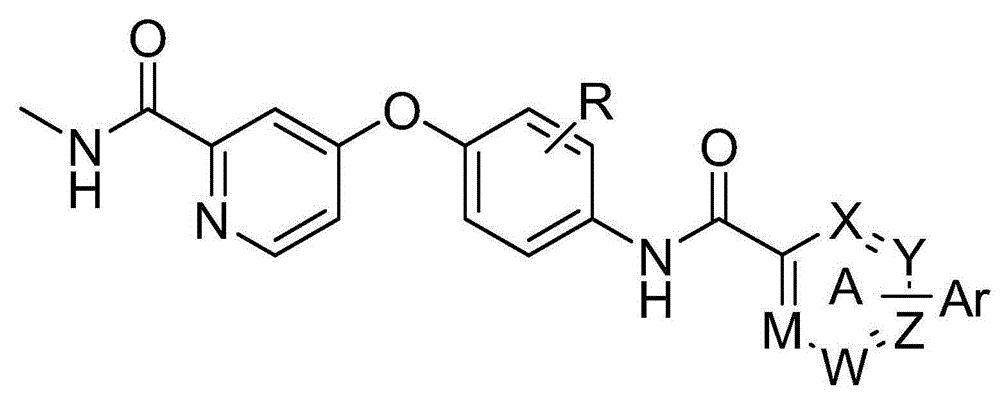
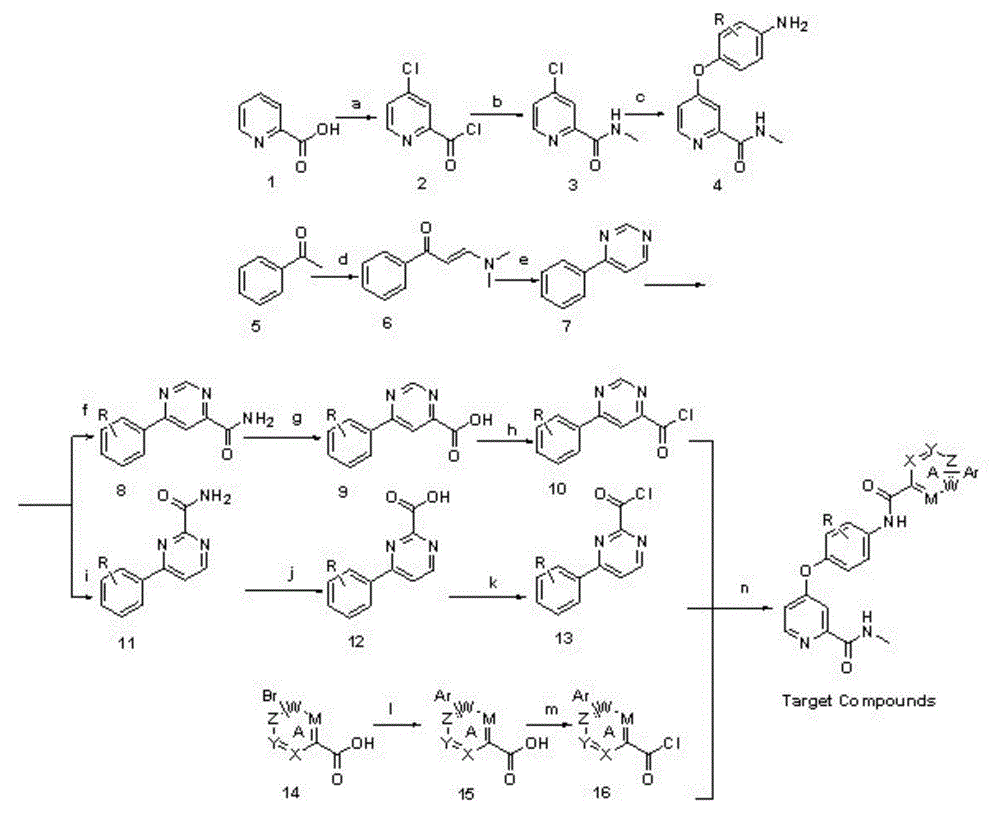
Biaryl amide structure containing sorafenib derivative as well as preparation method and applications thereof
A technology for arylamides and derivatives, applied in the field of sorafenib derivatives containing biarylamide structure and its preparation, can solve the problems of no inhibitory activity and weak effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] N-(4-(2-(Methylcarbamoyl)pyridin-4-yloxy)phenyl)-4-p-chlorophenylpyridinamide
[0099] Step a4-chloropicolinate chloride (formula 2)
[0100] Put 10g (0.081mol) of 2-pyridinecarboxylic acid, 1.3g (0.013mol) of sodium bromide and 13mL of chlorobenzene in a 250mL three-necked flask, after heating up to 50°C, slowly add 24mL of thionyl chloride dropwise, and the dropwise addition is complete Continue stirring for 3 minutes, heat up to 85°C, and reflux for 20 hours. After the reaction was completed, the solvent and excess thionyl chloride were evaporated under reduced pressure, and 15 mL of toluene was added and stirred for 5 minutes. After the solvent was evaporated, a yellow oily liquid was obtained, namely 4-chloropicolidinyl chloride, and 50 mL of toluene was added, and the solution was directly used react in the next step.
[0101] Step b4-chloro-N-picoline carboxamide (formula 3)
[0102] Put 30mL of 33% methylamine aqueous solution into a 250mL round-bottomed flas...
Embodiment 2
[0116] N-(4-(2-(Methylcarbamoyl)pyridin-4-yloxy)phenyl)-4-p-tolylpyridinamide
[0117] ESI-MS [M+Na] m / z: 461.1; 1 HNMR(400MHz,DMSO)δ10.92(s,1H),8.80(d,J=4.9Hz,2H),8.53(d,J=5.5Hz,1H),8.42(s,1H),8.10(d, J=8.7Hz, 2H), 8.01(s, 1H), 7.82(d, J=7.8Hz, 2H), 7.43(s, 1H), 7.40(d, J=7.6Hz, 2H), 7.27(d, J=8.6Hz, 2H), 7.20(s, 1H), 2.80(d, J=4.5Hz, 3H), 2.40(s, 3H).
Embodiment 3
[0119] N-(4-(2-(Methylcarbamoyl)pyridin-4-yloxy)phenyl)-4-phenylpyridineamide
[0120] ESI-MS [M+K] m / z: 463.2; 1 HNMR(400MHz,DMSO)δ10.91(s,1H),8.82(t,J=4.5Hz,1H),8.79(d,J=4.8Hz,1H),8.52(d,J=5.6Hz,1H) ,8.43(d,J=1.3Hz,1H),8.09(d,J=9.0Hz,2H),8.02(dd,J=5.1,1.8Hz,1H),7.94–7.88(m,2H),7.56( ddd,J=10.8,9.8,5.4Hz,3H),7.42(d,J=2.5Hz,1H),7.26(d,J=9.0Hz,2H),7.18(dd,J=5.6,2.6Hz,1H ),2.79(d,J=4.8Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com