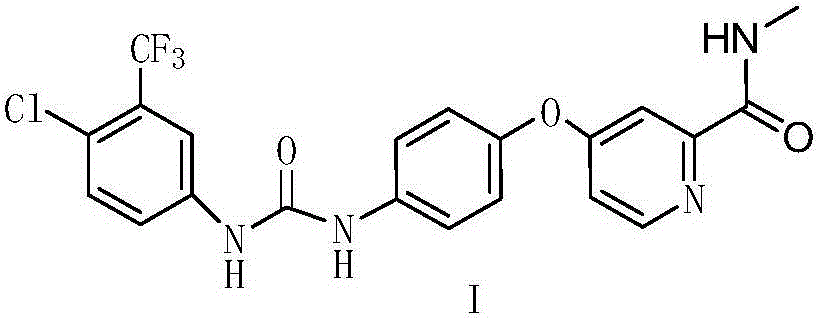
Novel method for synthesizing sorafenib
A sorafenib crude product and compound technology, which is applied in the preparation of sorafenib and the field of pharmaceutical compound preparation, can solve the problems of violent reaction, uncontrollable reaction, and use of highly toxic raw materials, etc., and achieve the effect of simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
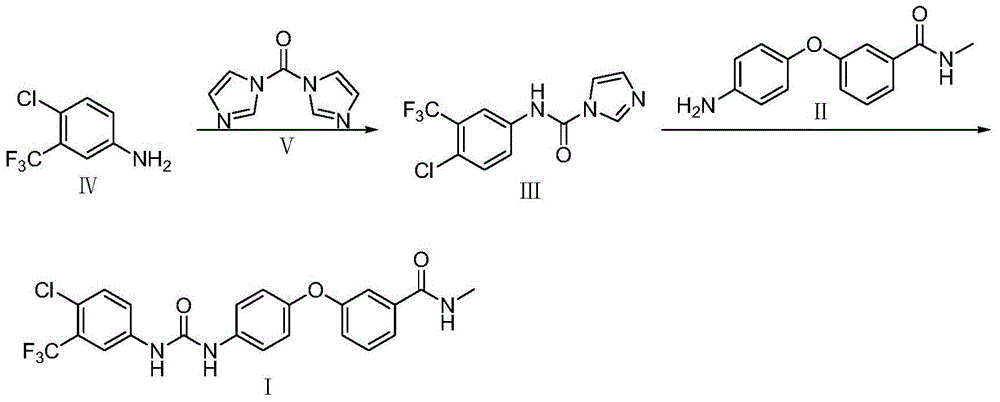
[0033] Example 1 Preparation of compound III (4-chloro-3-trifluoromethylphenylcarbamoyl imidazole)
[0034] Dissolve 2.0kg of compound IV (4-chloro-3-trifluoromethylaniline) in 17.0L of 1,2-dichloroethane, stir, add 2.3kg of compound V (N,N'-carbonyldiimidazole), Raise the temperature to 50°C, react for 10h, and then transfer to room temperature for 1h. Filter, wash twice with 1,2-dichloroethane, filter dry, and vacuum-dry at 40°C to obtain 1.6kg of compound III (4-chloro-3-trifluoromethylphenylcarbamoyl imidazole) with a purity of 99.2% , yield 85%.
Embodiment 2
[0035] Embodiment 2 prepares Sorafenib crude product
[0036] Add 200L of ethyl acetate and 18.7kg of compound III to the reaction tank, stir and raise the temperature to 25°C, then add 9.8kg of compound II, keep stirring at 25°C for 3h, cool down to 20°C to grow crystals for 2h after the reaction, filter, and use acetic acid Wash twice with ethyl ester, filter dry, and vacuum-dry at 40°C to obtain 17.2 kg of crude sorafenib with a purity of 99.18% and a yield of 91.1%.
Embodiment 3
[0037] Embodiment 3 prepares Sorafenib crude product
[0038] Add 200L of dichloromethane and 18.7kg of compound III to the reaction tank, stir and raise the temperature to 35°C, then add 9.8kg of compound II, keep stirring at 35°C for 2h, cool down to 20°C to grow crystals for 2h after the reaction, filter, and use two Wash twice with methyl chloride, filter dry, and vacuum-dry at 40° C. to obtain 16.9 kg of crude Sorafenib with a purity of 99.20% and a yield of 90.22%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com