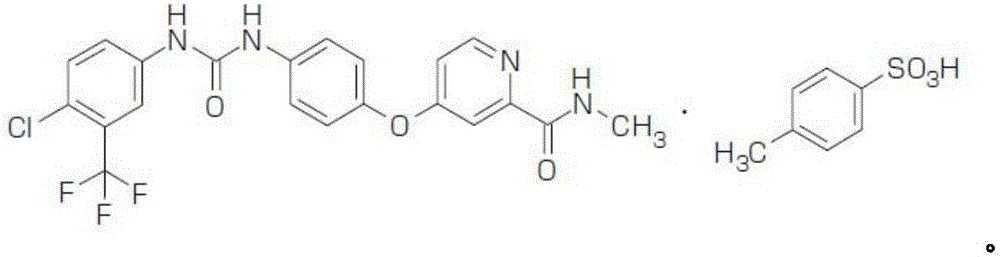
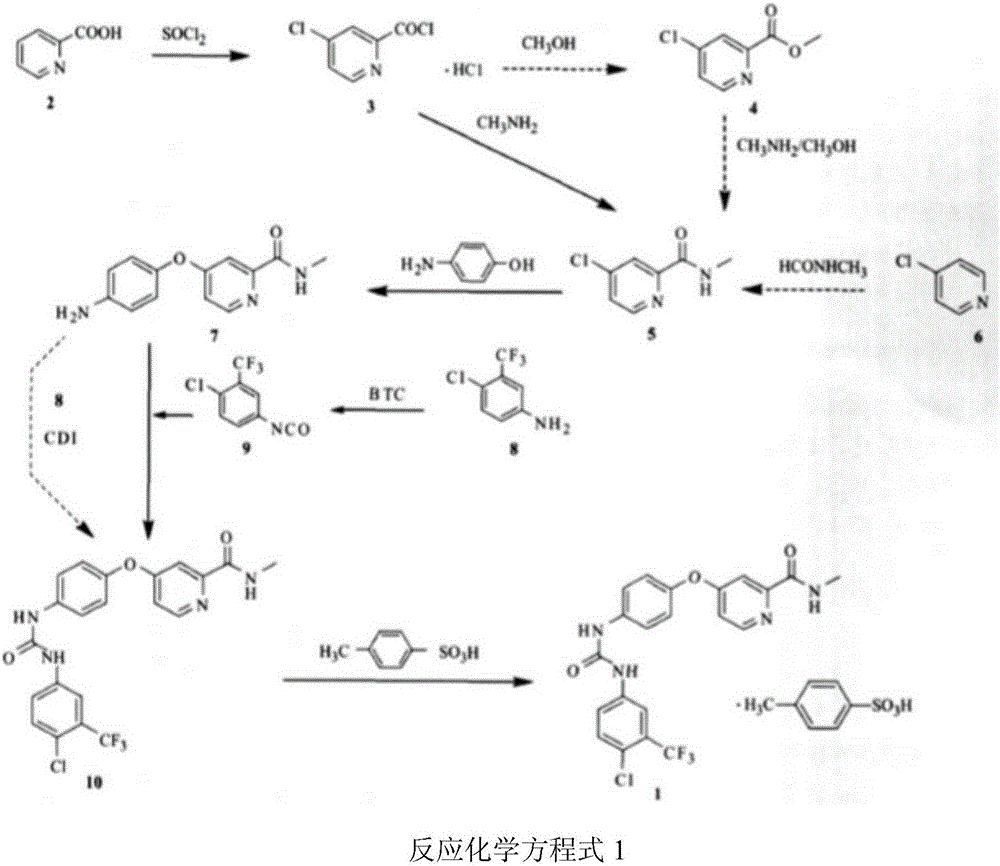
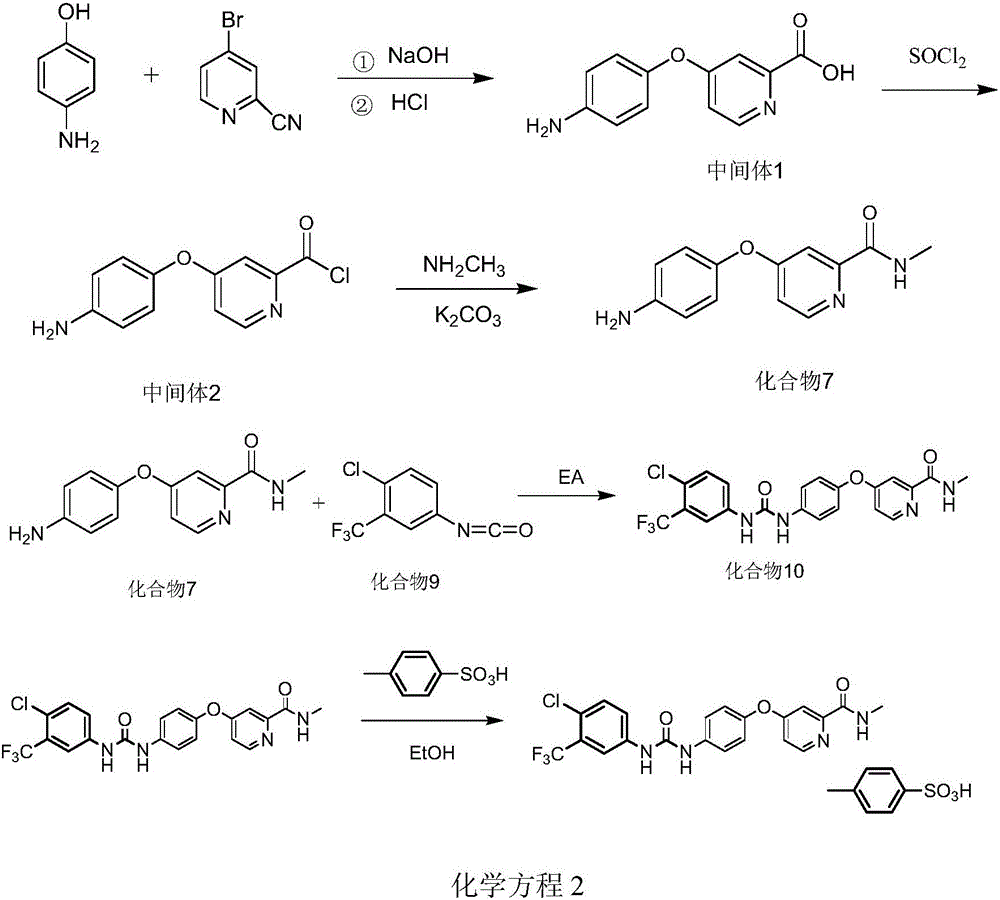
Method for preparing sorafenib tosylate
A technology of toluenesulfonic acid and p-toluenesulfonic acid, which is applied in the field of medicine, can solve the problems of low yield and complicated steps, and achieve the effects of high yield, simple method and simple reaction steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1: the preparation of compound 7
[0031] (1) 183g (1mol) of 4-bromo-2-cyanopyridine was dissolved in 1L of tetrahydrofuran, and 118g (1.08mol) of p-aminophenol and 50% sodium hydroxide solution (70g of sodium hydroxide+70ml of water) were added; Heat to reflux for 5.5 hours; heat to remove tetrahydrofuran; add 1L of water to the concentrated solution, then add concentrated hydrochloric acid to adjust the pH to 5.5, a brown solid gradually precipitates out, suction filters, and the filter cake is blown-dried to obtain 204.2 g of a brown solid, which is Intermediate 1 (for the next step with a purity of 100%). Mass spectrometry ESI-MS gives the molecular ion peak of intermediate 1 as 231 [M+H] + .
[0032] (2) Add 204.2g of intermediate 1 into 500ml of chloroform, then add 260g (2.18mol) of thionyl chloride, heat and reflux for 1.5 hours, and then directly evaporate to dryness (distilled under reduced pressure at 60°C, if necessary, increase The temperature...
Embodiment 2
[0034] Embodiment 2: the preparation of compound 7
[0035](1) Dissolve 1830g of 4-bromo-2-cyanopyridine in 8L of tetrahydrofuran, add 1150g of p-aminophenol and 50% sodium hydroxide solution (650g of sodium hydroxide + 650ml of water); heat to reflux for 6 hours; heat to evaporate Tetrahydrofuran; add 9L of water to the concentrated solution, then add concentrated hydrochloric acid to adjust the pH5.5, brown solids gradually precipitate out, filter with suction, and after the filter cake is air-dried, 2038g of brown solids are obtained, which is intermediate 1 (carried out according to the purity of 100%) step reaction). Mass spectrometry ESI-MS gave the molecular ion peak of intermediate 1 as 231[M+H]+.
[0036] (2) Add 2038g of intermediate 1 into 4.5L of chloroform, then add 2500g of thionyl chloride, heat and reflux for 1.5 hours, then directly evaporate to dryness (distill under reduced pressure at 60°C, raise the temperature and continue heating if necessary, No resid...
Embodiment 3
[0038] Embodiment 3: the preparation of Sorafenib free base
[0039] 1600 g of compound 7 (Example 2) was dissolved in 6400 mL of ethyl acetate, and stirred evenly at room temperature to obtain a suspension. Dissolve 1604.8g of compound 9 (4-chloro-3-(trifluoromethyl)phenylisocyanate) in 1600mL of ethyl acetate (endothermic); after stabilization, add dropwise to the above-mentioned compound 7 in ethyl acetate solution , temperature control temperature 20 ~ 30 ℃, 30min-60min dripping. During the dropwise addition, the suspension gradually became clear, and then a large amount of precipitate precipitated out. After the drop was completed, it was stirred at room temperature for 4 hours. After suction filtration, the filter cake was washed with ethyl acetate to obtain a light brown powder.
[0040] The light brown powder was recrystallized by adding 21L of absolute ethanol, heated to reflux to dissolve, then lowered to room temperature and stirred for 1-2 hours to crystallize. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com