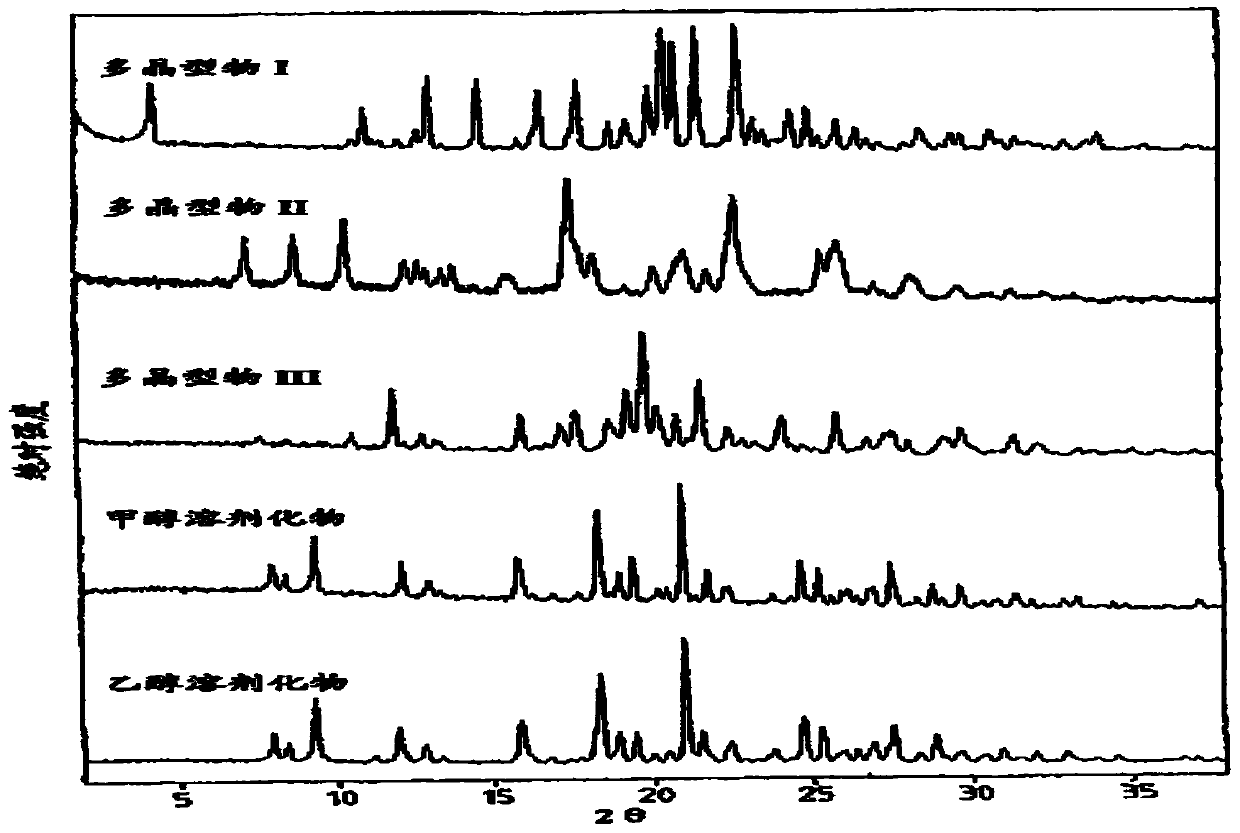
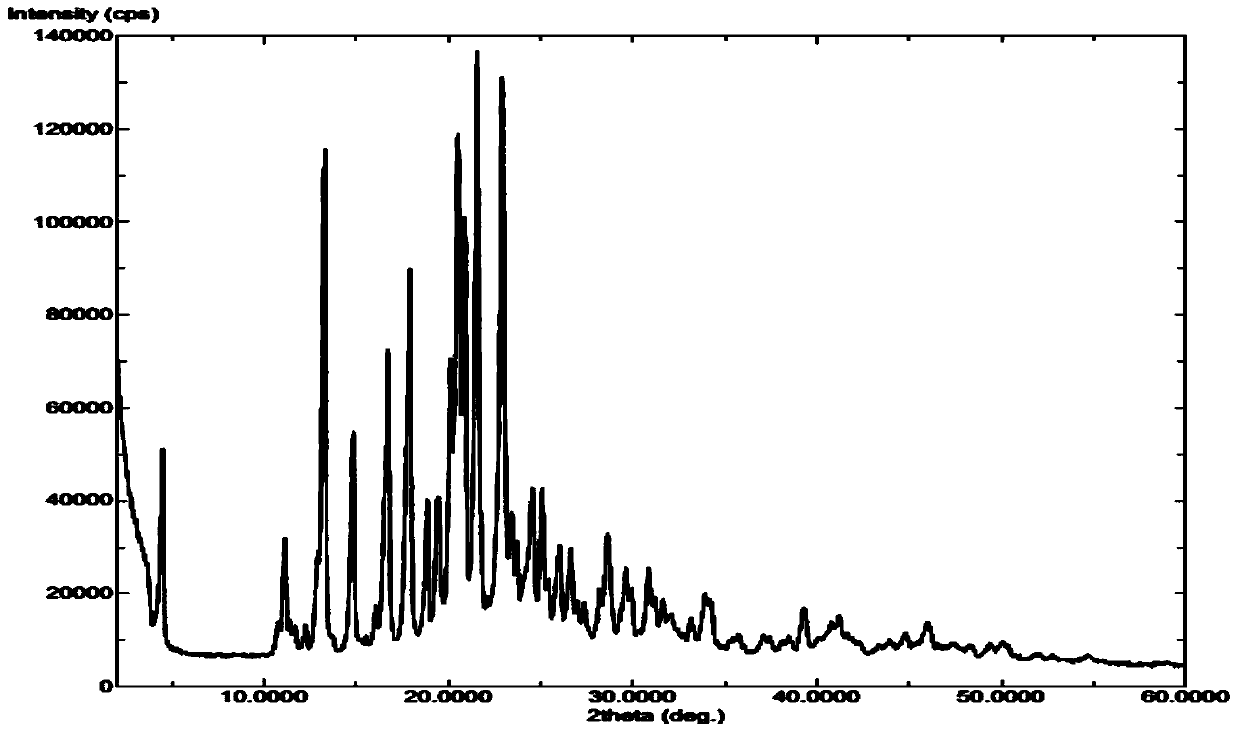
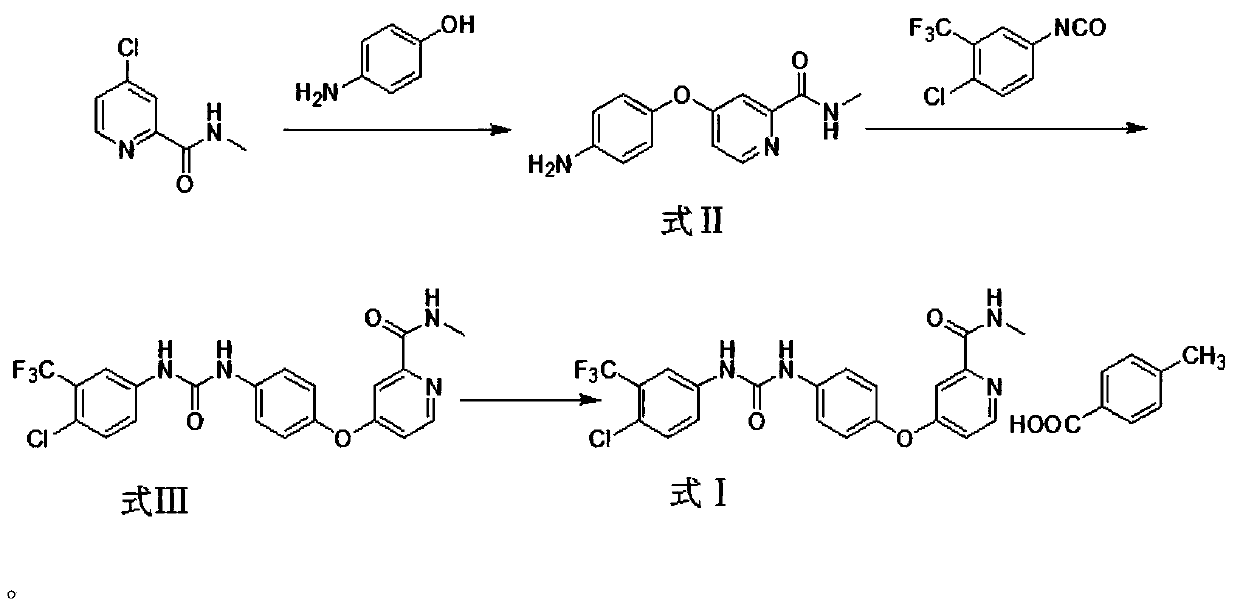
Method for industrial production of sorafenib tosylate polymorphic form I
A technology of polymorphic form and toluenesulfonic acid of fenib, which is applied in the field of industrial production of polymorphic form I of sorafenib tosylate, can solve problems such as unfavorable scale-up production, cumbersome conversion operations, etc., and achieves low pollution and short process route. , the effect of high product purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Synthesis of Embodiment 1 Sorafenib Tosylate Polymorph I
[0033] ①Synthesis of Compound 1
[0034] In a 50L reaction kettle, add 12L of nitrogen, nitrogen-dimethylformamide, and add 1.92kg (17.58mol) of p-aminophenol and 960g of sodium hydroxide into the reaction flask. Then, under nitrogen protection, 300 g of anhydrous sodium carbonate was added, 3.0 kg (17.58 mol) of N-methyl-4-chloro-pyridineformyl was added into 12 L of DMF to dissolve, and N-methyl-4-chloro-pyridineformyl Acyl DMF solution was added dropwise into the reaction system. After the dropwise addition was completed, stir at room temperature for 30 min. The reaction solution was heated to 90°C, stirred for 4 hours, and the reaction of the raw materials was complete. After the reaction, 16L of water was added to the system, extracted with ethyl acetate, the organic layer was concentrated and dried, beated with petroleum ether for 1 hour, then suction filtered, the filter cake was washed with petroleum ...
Embodiment 2
[0040] Synthesis of Embodiment 2 Sorafenib Tosylate Polymorph I
[0041] ①Synthesis of Compound 1
[0042]In a 50L reactor, add 12L of nitrogen, nitrogen-dimethylformamide, 3.84kg (35.16mol) of p-aminophenol and 960g of sodium hydroxide into the reaction flask. Then, under the protection of nitrogen, 3.0kg (17.58mol) of N-methyl-4-chloro-pyridinecarboxamide was added to 12L of DMF to dissolve, and the DMF solution of N-methyl-4-chloro-pyridinecarboxamide was added dropwise to in the reaction system. After the dropwise addition was completed, stir at room temperature for 30 min. The reaction solution was heated to 100°C and stirred for 4 hours. After the reaction, 16L of water was added to the system, extracted with ethyl acetate, the organic layer was concentrated and dried, beated with petroleum ether for 1 hour, then suction filtered, the filter cake was washed with petroleum ether, and dried by blasting to obtain tan solid compound 1, 2.2kg, collected rate of 63%.
[0...
Embodiment 3
[0048] Synthesis of Embodiment 3 Sorafenib Tosylate Polymorph I
[0049] ①Synthesis of Compound 1
[0050] In a 50L reactor, add 12L nitrogen, nitrogen-dimethylformamide, add 4.80kg (43.95mol) of p-aminophenol, and 960g of sodium hydroxide into the reaction flask. Then, under nitrogen protection, 300 g of anhydrous sodium carbonate was added, 3.0 kg (17.58 mol) of N-methyl-4-chloro-pyridinecarboxamide was added to dissolve in 12 L of DMF, and N-methyl-4-chloro- The DMF solution of picolilyl was added dropwise into the reaction system. After the dropwise addition was completed, stir at room temperature for 30 min. The reaction solution was heated to 110°C and stirred for 4 hours. After the reaction, 16L of water was added to the system, extracted with ethyl acetate, the organic layer was concentrated and dried, beated with petroleum ether for 1 hour, then suction filtered, the filter cake was washed with petroleum ether, and dried by blast to obtain tan solid compound 1, 2.8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com