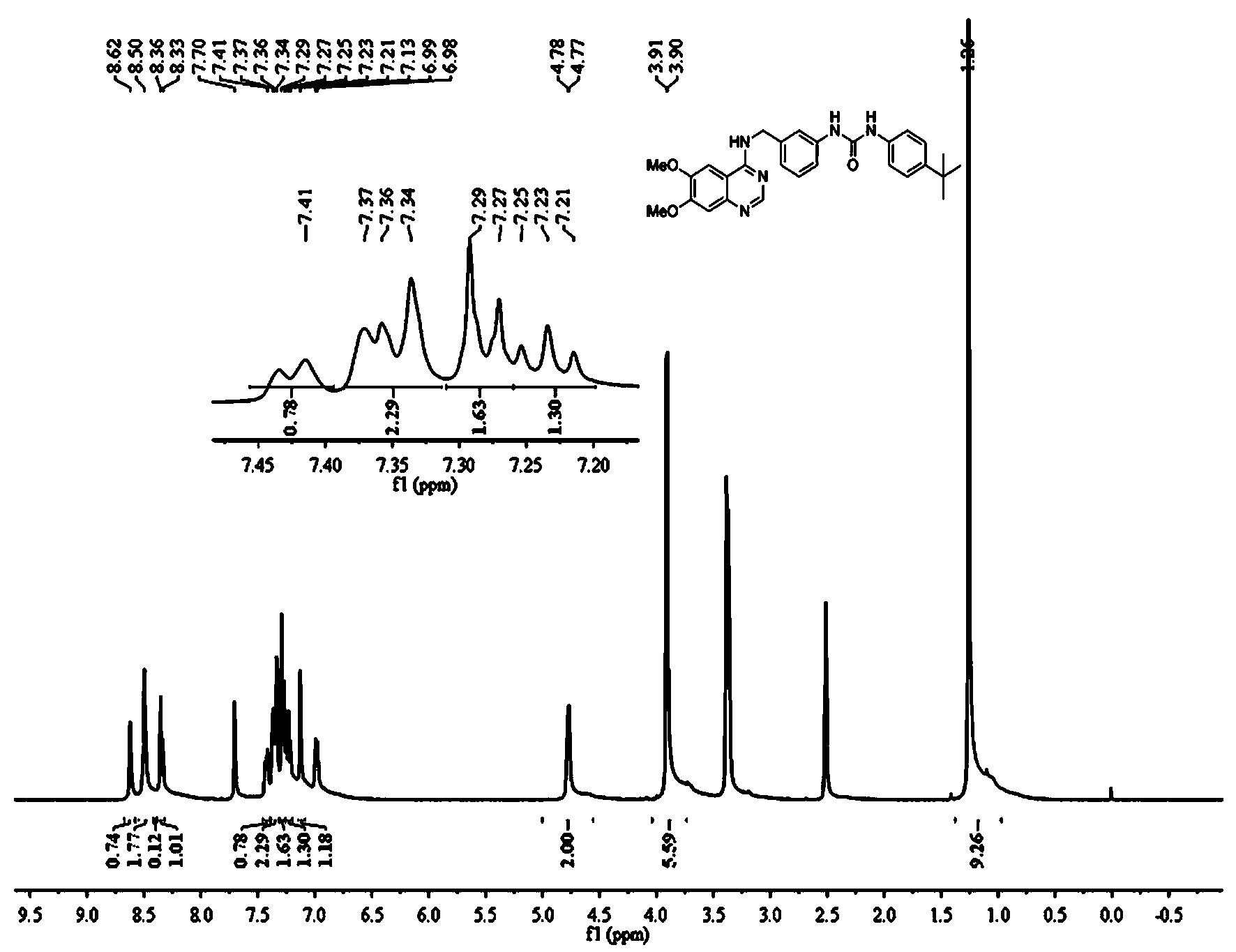
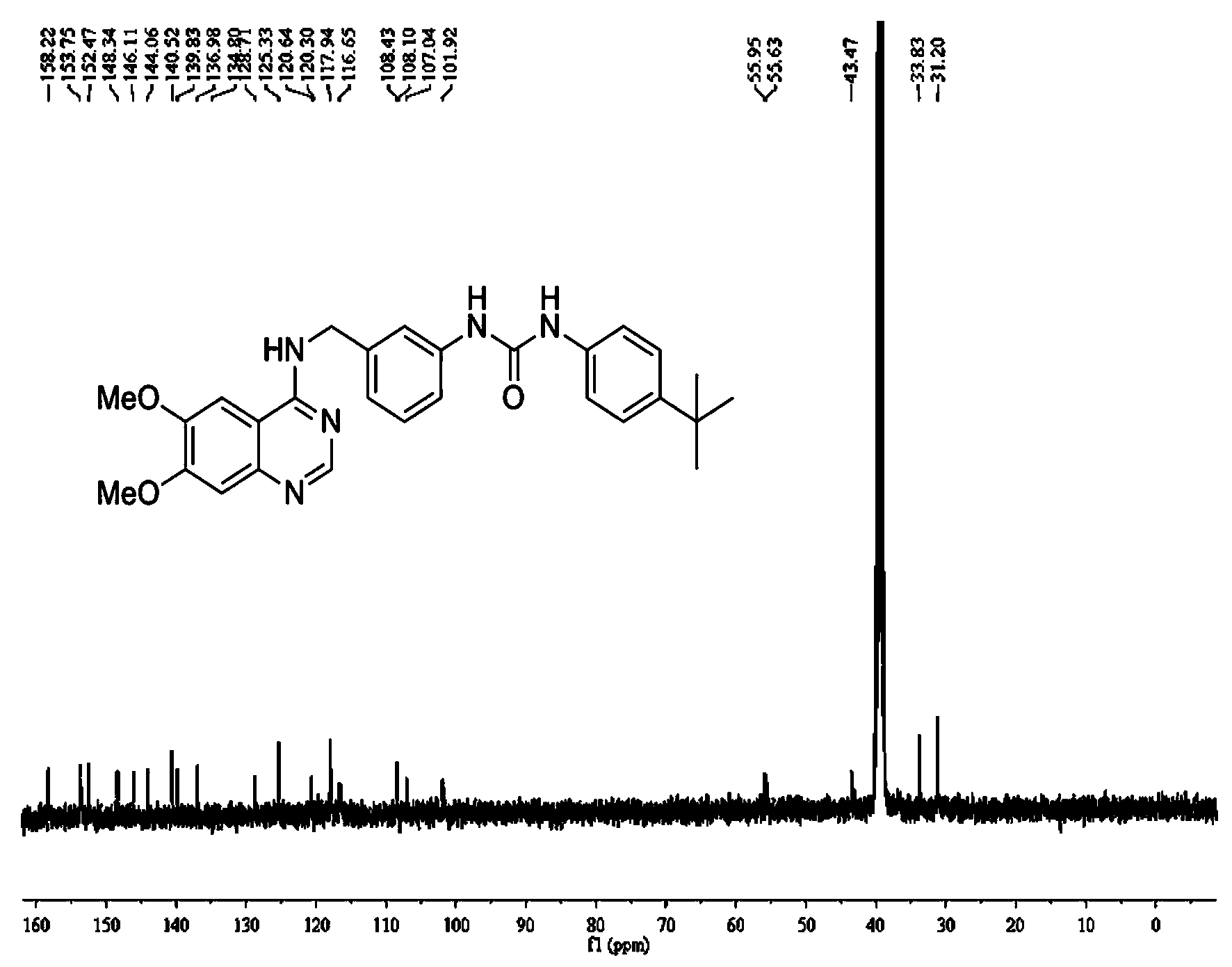
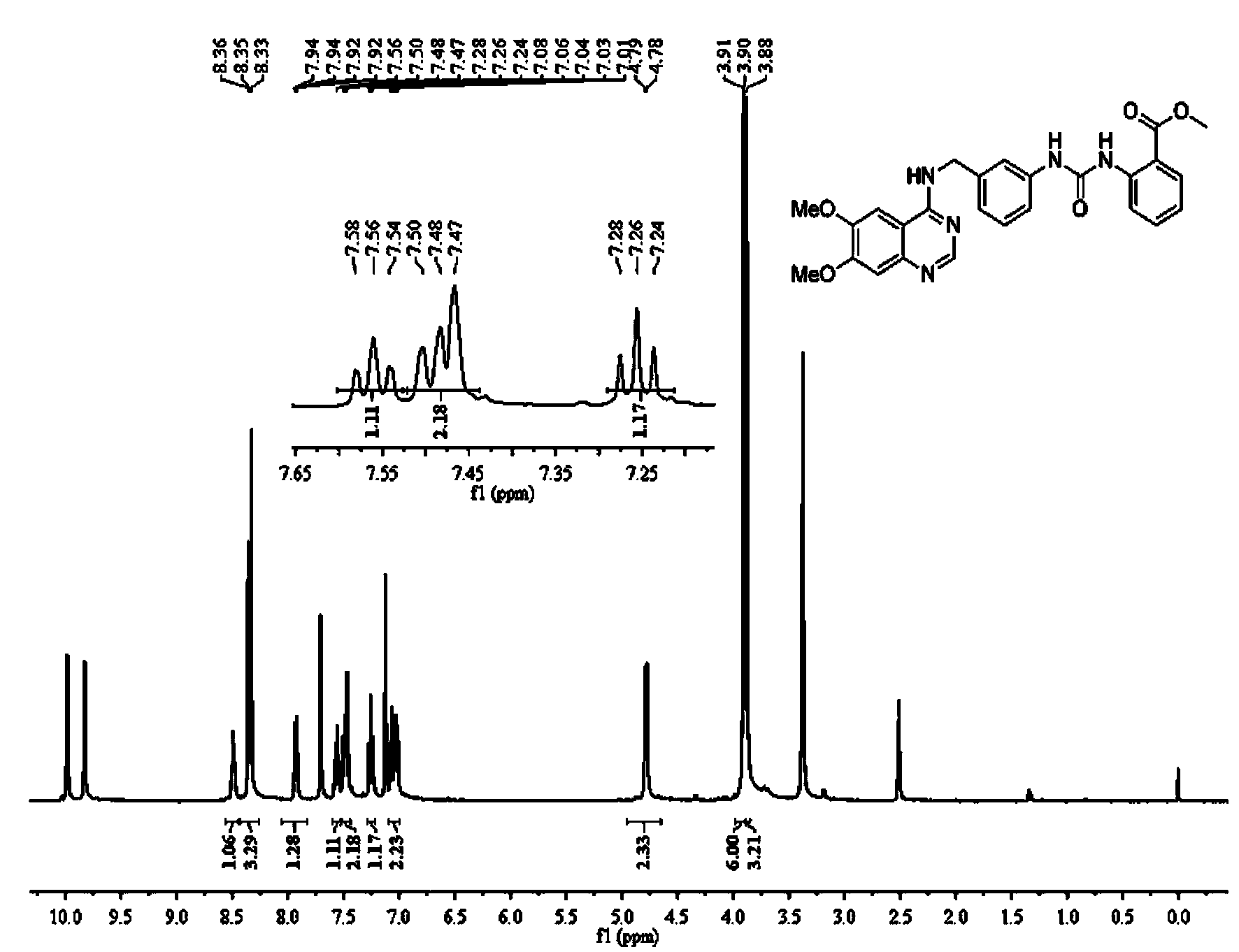
Quinazolinyl-aryl urea derivatives with antitumor function and application thereof
A technology of aryl urea derivatives and quinazoline, which is applied in the field of medicine, can solve the problems of tumor cell line induction of apoptosis, influence of intracellular active oxygen levels, and insufficient research
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0139] Preparation of isocyanate series compounds
[0140] For different isocyanates, it is advisable to use one of the following two methods to synthesize them: if the boiling point of the isocyanate is low, it can be prepared by general method one; and for the higher boiling point, it can be obtained by general method two. The prepared isocyanate is easy to absorb moisture in the air and hydrolyzes, so it is not suitable for further purification, and can be stored dry or used directly in the reaction.
[0141] General method 1: Add triphosgene (BTC, 18mmol, 0.5eqv.) and solvent dichloroethane (DCE, 20mL) to a 250mL flask in an ice bath and stir. After dissolving, slowly add Aniline or its derivatives (36 mmol) in DCE solution (10 mL), keep the temperature of the reaction system at 0-5°C. The dropwise addition time is about 1 hour. At this time, the reaction system becomes turbid. After the dropwise addition, it rises to room temperature and reacts for 1 hour, and then heats...
Embodiment 2-39
[0180] The structure of each compound in the quinazoline-aryl urea derivatives is shown in Table 1, including two series of products in Table 1, series I: N-substituted phenyl-N'-{3-[(6,7 -Dimethoxy-4-aminoquinazoline)benzyl]}urea, including compound I-1~compound I-19; series II: N-substituted phenyl-N'-{3-[(4-amino Quinazoline) benzyl]} urea, including compound II-1 to compound II-19.
[0181] The two series of product structural formulas that embodiment 2-39 obtains are as follows:
[0182]
[0183] Table 1
[0184]
[0185]
Embodiment 2
[0188] N-(2-methoxyphenyl)-N'-{3-[(6,7-dimethoxyquinazoline-4-amino)methyl]phenyl}urea (Compound I-1)
[0189] Step A: Preparation of 6,7-dimethoxyquinazolin-4-one
[0190] Add 2-amino-6,7-dimethoxybenzoic acid methyl ester (20g, 94.7mmol), formamide (160mL), formic acid (4mL) to a 250mL round bottom flask successively, reflux at 160°C for 10h, TLC ( V 乙酸乙酯 :V 石油醚 =1:1) to monitor the completion of the reaction, quickly pour the resulting mixture into ice water, stir, and let it stand for a while, the solid precipitates, is filtered by suction, washed with distilled water (3×15mL), ethyl acetate (3×15mL) ), dried, recrystallized from ethyl acetate, and dried to obtain off-white granular crystals, namely the product (16.44g, 84.22%). m.p.282.1~288.4℃. 1 H NMR (500MHz, DMSO-d 6 )δ(ppm):7.99(s,1H),7.45(s,1H),7.14(s,1H),3.91(s,3H),3.87(s,3H).ESI-HRMS(m / z): calcd.for C 10 h 10 N 2 o 3 [(M+H) + ], 207.07250; found, 207.07244.
[0191] Step B: Preparation of 4-chloro-6,7-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com