Preparation for improving bioavailability of sorafenib
A technology of sorafenib and preparations, which is applied in the field of preparations for improving the bioavailability of sorafenib, and can solve the problems to be further studied and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Example 1 Material
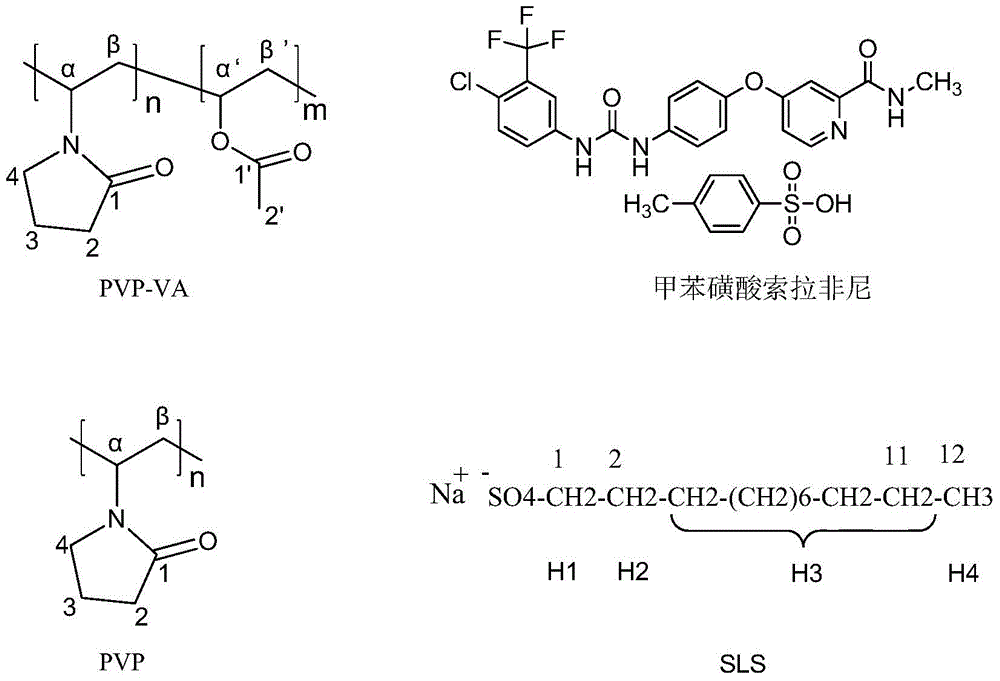
[0044] Sorafenib tosylate used in the embodiments of the present invention is provided by Qilu Pharmaceutical Co., Ltd. (Shandong, China); Polyvinylpyrrolidone PVP (Kollidon30) and polyvinylpyrrolidone vinyl acetate PVP-VA (KollidonVA64) are provided by German BASF Presented by the chemical reagent company. All salts used to dissolve the solution and methanol (analytical grade) used for spray drying were provided by Beijing Chemical Plant. The chemical structures and key features of the complexes and compounds mentioned in the present invention are given by figure 1 shown.
Embodiment 2
[0045] The influence of embodiment 2PVP-VA and PVP on the supersaturation of Sorafenib in FaSSIF
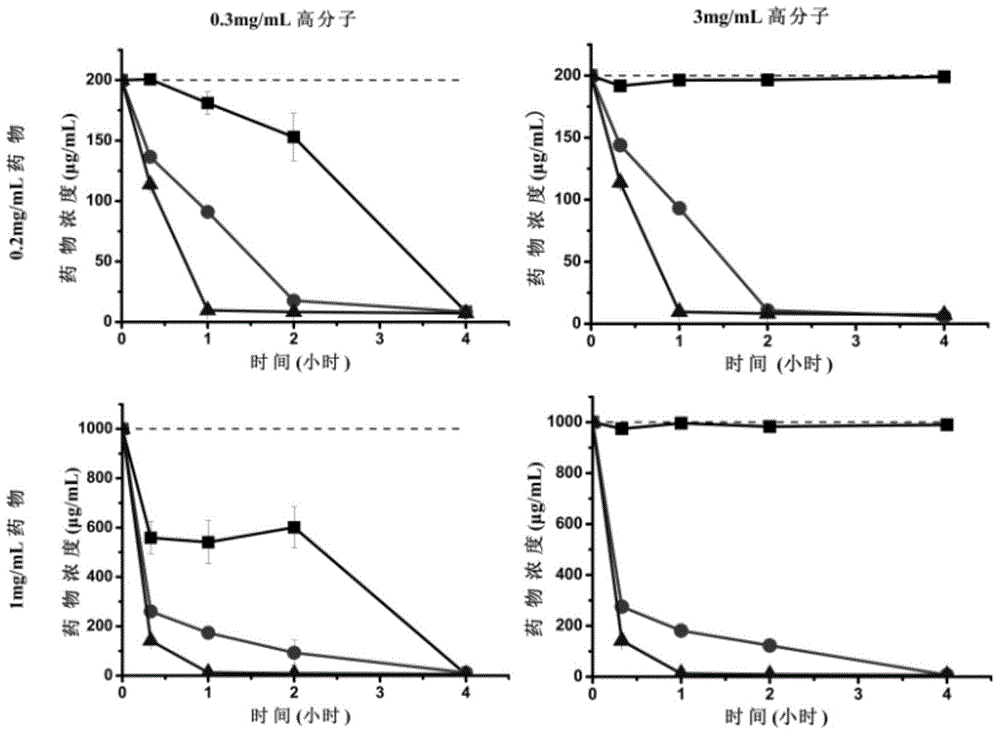
[0046] In the embodiments of the present invention, in order to evaluate the impact of the compound on maintaining the supersaturated state of Sorafenib, the polymer compound PVP-VA or PVP was dissolved in the simulated intestinal fluid FaSSIF at a concentration of 0.3 and 3 mg / mL, and toluenesulfonic acid Sorafenib was dissolved in DMSO at a concentration of 100 or 20 mg / mL. In the dissolution medium, 100 microliters of Sorafenib drug solution was added to 10mL of polymer compound solution. The obtained mixture solution was shaken and dissolved on a shaker (brand: Tianjin Uno, model: WE-2), the shaking speed was 100 rpm, and the temperature was 37 degrees Celsius. After 0.3, 1, 2 and 4 hours, the solution was centrifuged at 15000rpm for 3min, and the concentration of the drug in the supernatant was analyzed by high performance liquid chromatography (HPLC) (brand: ShimadzuLC-20A...
Embodiment 3
[0054] The influence of embodiment 3SLS on the dissolution behavior of Sorafenib
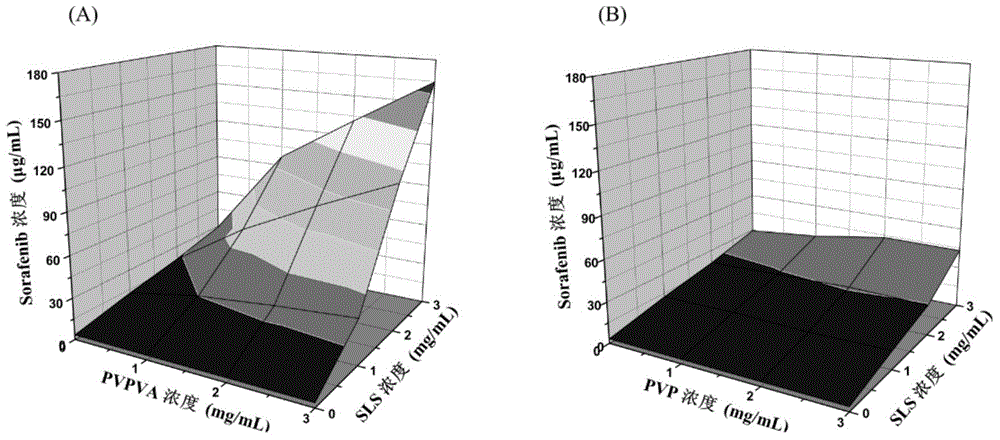
[0055] In this example, the inventors investigated the effects of PVP-VA, PVP-VA+SLS, PVP, PVP+SLS and SLS on the dissolution behavior of sorafenib. The results of the investigation are as follows: image 3(The concentration of sorafenib on the ordinate indicates the concentration of sorafenib) shows that the solubility of sorafenib in FaSSIF is 3.3 micrograms / mL, and when the concentration of SLS is increased to 3mg / mL alone, the solubility of sorafenib can reach 29.4 micrograms / mL . There was no significant change in the solubility of sorafenib when increasing the polymer PVP-VA or PVP alone to 3 mg / mL. However, when 3mg / mL SLS and 3mg / mL PVP-VA co-exist, the solubility of Sorafenib increased to 164.6 μg / mL. However, this synergistic solubilization phenomenon was not obvious when PVP and SLS co-existed. When 3mg / mLPVP and 3mg / mLSLS co-exist, the solubility of sorafenib is only 43.3μg / mL. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com