Liver targeted medicine
A liver targeting and drug technology, applied in the field of biomedicine, can solve the problem of unpredictable connection between targeting molecules and drug molecules, and achieve the effect of improving liver targeting, improving drug targeting and high activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
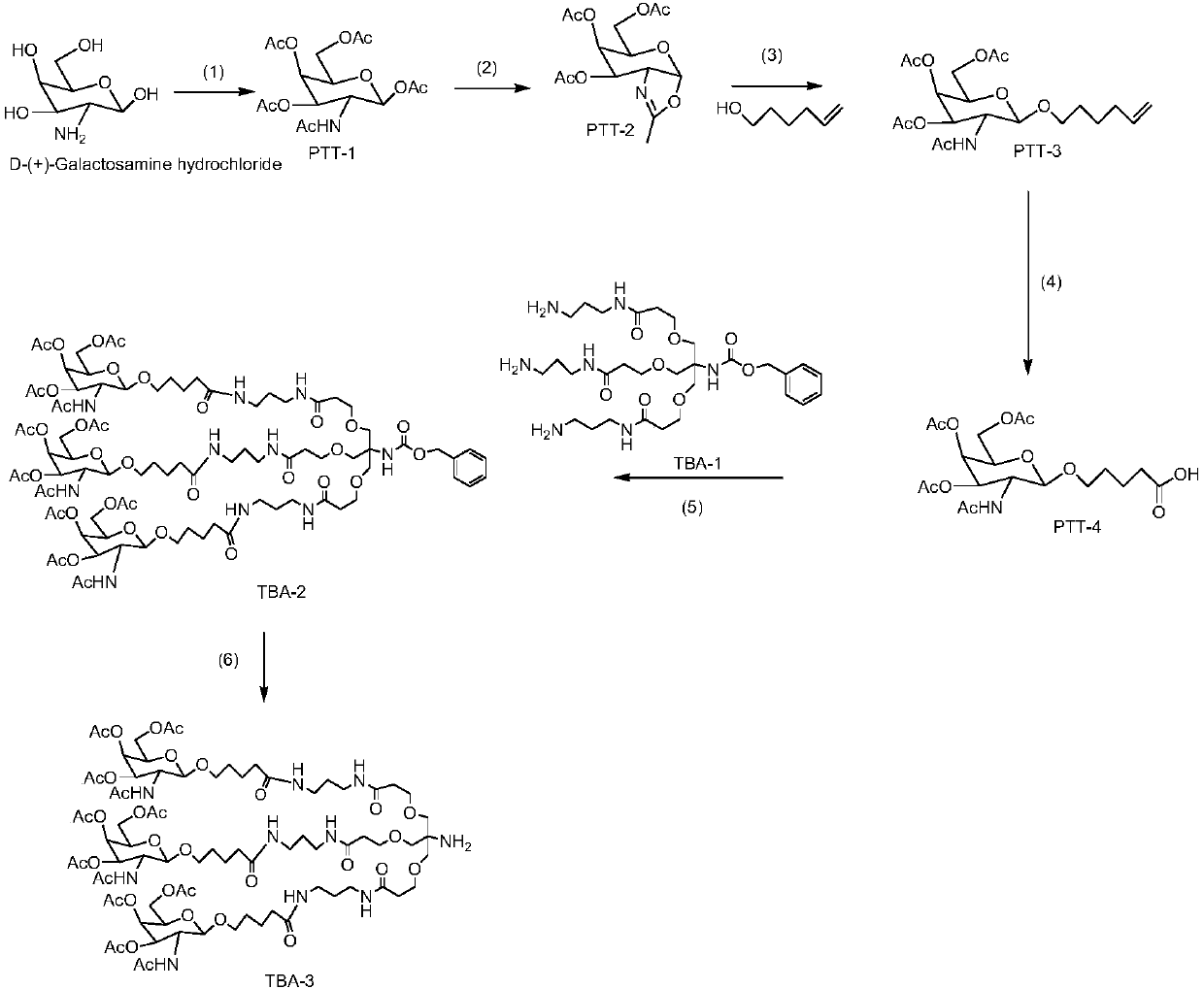
[0040] Preparation of trivalent acetylgalactosamine TBA-3
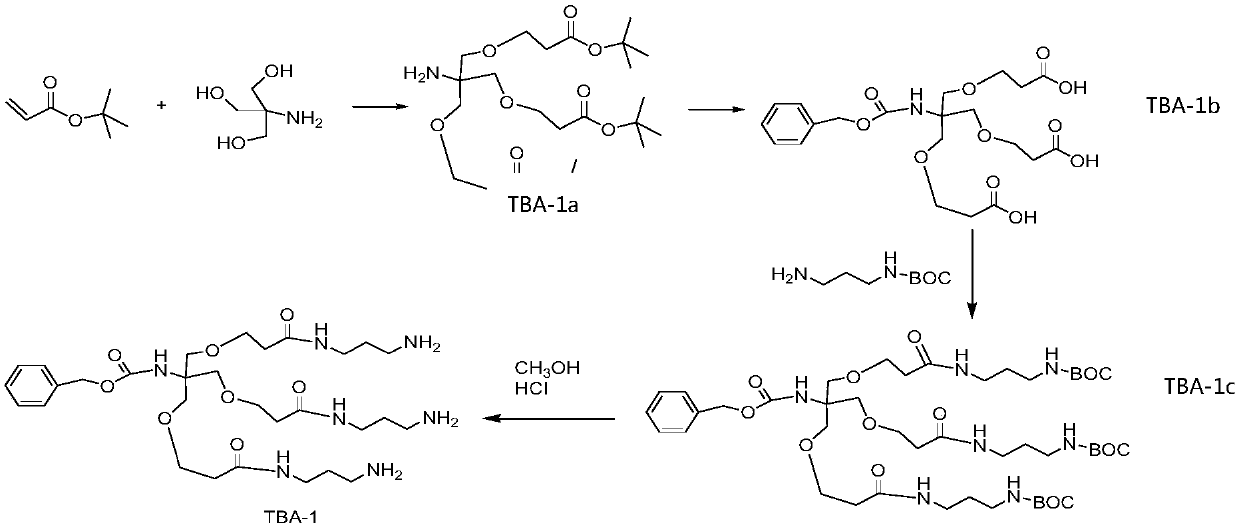
[0041] Prepare trivalent acetylgalactosamine TBA-3 with the steps in the following order, its structural formula and preparation flow chart are shown in figure 1 ,in:
[0042] (1) Preparation of compound PTT-1:
[0043]Take 10.0 g (55.8 mmol) of acetylgalactosamine hydrochloride (purchased from TCI (Shanghai) Chemical Industry Development Co., Ltd., product code: G0007), and add it to 100 mL of dichloromethane, and add 0.16 g ( 1.3mmol) of 4-dimethylaminopyridine (DMAP) and 20mL of triethylamine, after dissolving, start ice bathing; while ice bathing, add 60mL (635.2mmol) of acetic anhydride dropwise to it, after the dropwise addition, react at room temperature , after monitoring the reaction completely with thin layer chromatography (TLC); 100mL of water washes the organic layer, repeats 3 times, the organic layer is dried, evaporated to dryness under reduced pressure to obtain white solid 18.0g, and its structural...
Embodiment 2
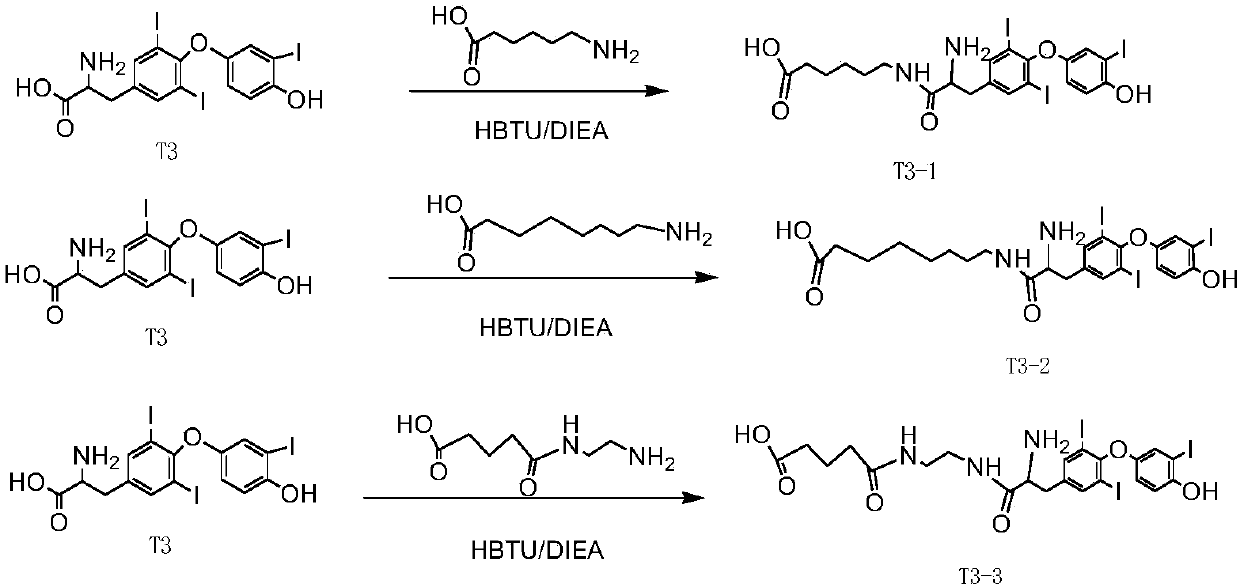
[0062] A liver targeting drug TBA-4, its structural formula is as follows Figure 4 As shown, the drug is trivalent acetylgalactosamine and thyroxine T3 (its structural formula is as follows Figure 4 Shown, represented by compound T3) is obtained by direct connection.
[0063] The preparation steps of TBA-4 are as follows: Take 0.38g (0.21mmol) of TBA-3, dissolve it with 20mL of dimethylformamide (DMF), then add 74.2mg (0.23mmol) of HBTU and 0.12mL (0.69mmol) DIEA, stirred for 5 min; then added compound T3 (0.15 g, 0.23 mmol) dissolved in 10 mL of DMF (purchased from TCI (Shanghai) Chemical Industry Development Co., Ltd., product code: T0453), stirred overnight at room temperature to obtain a reaction Liquid A; Evaporate the reaction liquid A under reduced pressure. 3 and 25 mL of water; the organic layer was washed with anhydrous Na 2 SO 4 Dry, evaporate to dryness under reduced pressure, add 5mL methylamine ethanol solution, react at room temperature for 2h; evaporate t...
Embodiment 3
[0065] A liver targeting drug TBA-5, its structural formula is as follows Figure 4 As shown, the drug is obtained by connecting trivalent acetylgalactosamine and thyroxine T3 through a straight chain.
[0066] The preparation steps of TBA-5 are as follows: Take 0.38g (0.21mmol) of TBA-3, dissolve it with 20mL of DMF, then add 74.2mg (0.23mmol) of HBTU and 0.12mL (0.69mmol) of DIEA, stir the reaction for 5min; then add With the compound T3-1 (0.15g, 0.23mmol) dissolved in 10mL DMF, its structural formula is as follows Figure 4 shown), stirred and reacted at room temperature overnight to obtain reaction solution A; the reaction solution A was evaporated to dryness under reduced pressure. 3 and 25 mL of water; the organic layer was washed with anhydrous Na 2 SO 4 Dry, evaporate to dryness under reduced pressure, add 5mL methylamine ethanol solution, react at room temperature for 2h; evaporate the solvent under reduced pressure, and purify by silica gel column chromatography ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com