Galactose ligands and their application in liver-targeted liposomes
A technology of galactose ligand and phospholipid, which is applied in the field of medicine, can solve the problems of lowering liposome zeta potential, liposome instability, and many steps in chemical synthesis, and achieves good biodegradability and good biocompatibility The effect of simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] Embodiment 1: Reaction raw material: cholesterol, divinyl sebacate, lactitol
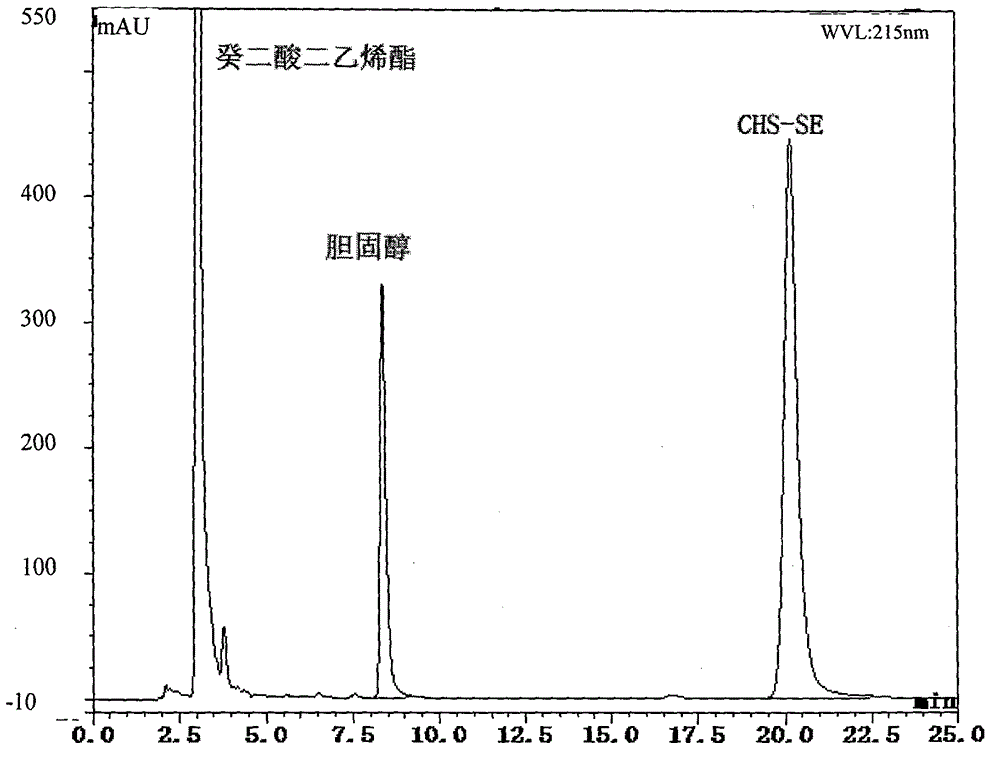
[0070] Step 1: Take a 10mL Erlenmeyer flask with a stopper, add 0.224mmol of divinyl sebacate and 0.025mmol of cholesterol, dissolve it with 5mL of dehydrated isooctane ultrasonically, shake it in an air bath constant temperature oscillator for 30min, and add 7.7mg of enzyme RCL / mL reaction system, start the reaction, the reaction temperature is 35°C, after the reaction for 11.47h, the enzyme is recovered by filtration, the filtrate is low-temperature vacuum to remove isooctane, and then ultrasonically dissolved with 20 times the amount of methanol, and placed at 0°C for 24h. When a large amount of crystals were precipitated, low-temperature rapid suction filtration was performed to obtain the intermediate product (II) as a white powdery solid with a yield of 92%. The reaction process is shown below:
[0071]
[0072] Structural characterization of intermediate product (II):
[0073] MS...
Embodiment 2
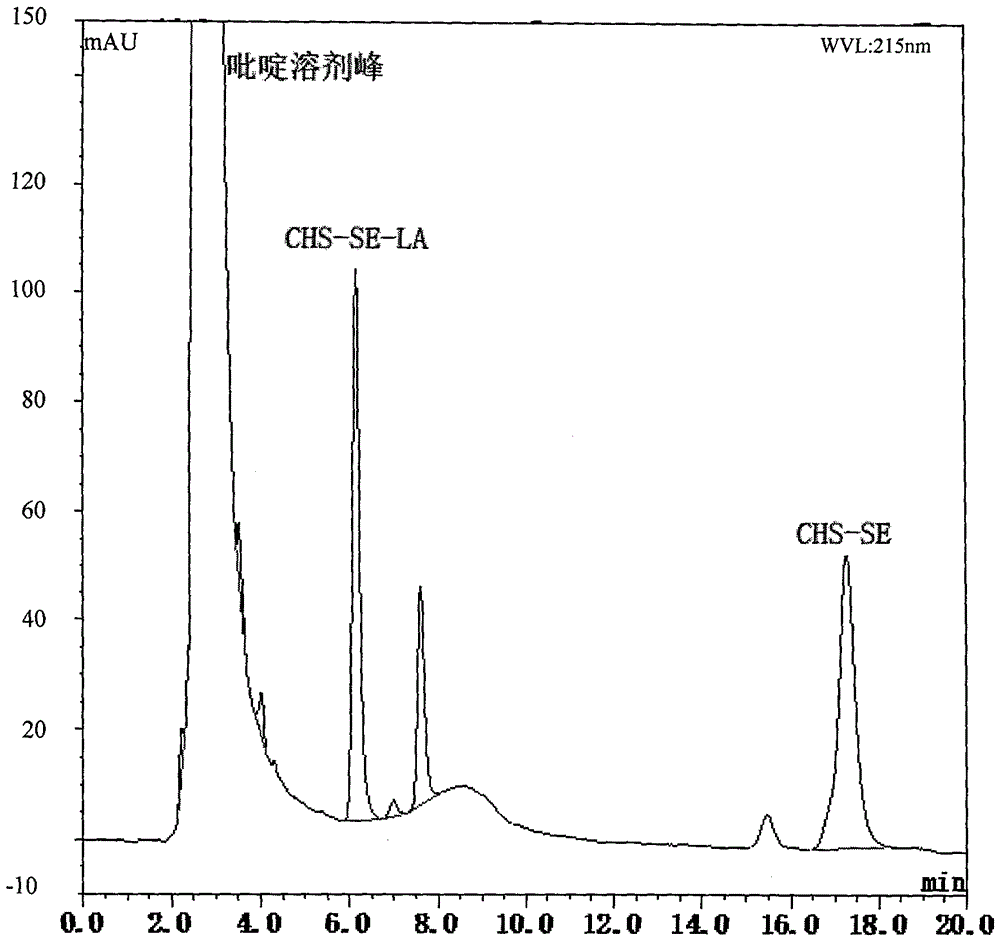
[0081] Embodiment 2: fully according to the method of embodiment 1, just change the divinyl sebacate used into H 2 C=CH-OOC-(CH2) 11 -COO-CH=CH 2 , the enzyme in step 1 was changed to Chirazyme L-2 lipase, with an amount of about 5 mg / mL, and the solvent was changed to acetone; and the enzyme in step 2 was changed to adopt commercial immobilized enzyme Novozym435, with an amount of about 30 mg, and the solvent was acetone: Pyridine 1:5; The product obtained is in the form of a white waxy solid with a total yield of 83.26%, and the structure is:
[0082]
[0083] The structure of the target product is characterized by:
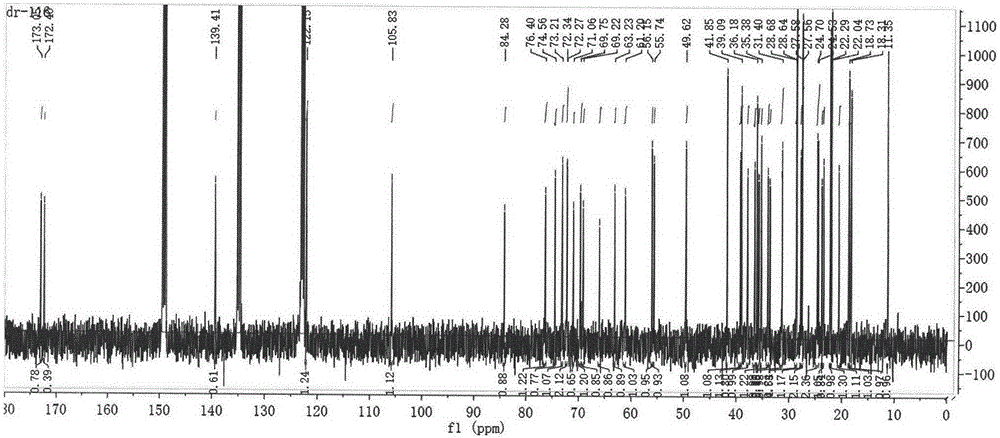
[0084] MS: [M+Na]+: 961.3; 13 C NMR (101MHz, Pyridine-d5) δ173.06, 172.40, 139.40, 122.16, 105.88, 84.38, 79.08, 76.42, 74.57, 73.21, 72.35, 72.28, 71.08, 69.76, 69.23, 65.11, 61.205, 66 ,49.62,41.85,39.31,39.09,37.97,36.61,36.19,35.85,35.39,34.15,33.75,31.50,31.40,29.14,29.05,28.90,28.74,27.85,27.58,24.77,24.59,23.85,23.51,22.29,22.05 , 20.64, 18.74, 1...
Embodiment 3
[0085] Embodiment 3: completely according to the method and condition of embodiment 1, just change the divinyl sebacate used into H 2 C=CH-OOC-(CH 2 ) 13 -COO-CH=CH 2 , the enzyme in step 1 was changed to Lipase QLM (Lipase QLM, a kind of lipase), the dosage was about 10 mg / mL, and the solvent was changed to petroleum ether; and the enzyme in step 2 was changed to commercial immobilized enzyme Novozym435 , the dosage is about 35 mg, and the solvent is pyridine+THF (1:1); the obtained product is in the form of a white waxy solid, with a total yield of 80.59%, and the structure is:
[0086]
[0087] Structural characterization of the target product:
[0088] MS: [M+Na]+ peak: 990.1; 13 C NMR (101MHz, Pyridine-d5) δ173.09, 172.43, 139.40, 122.17, 105.88, 84.36, 76.44, 74.59, 73.22, 72.37, 71.10, 69.77, 69.24, 66.13, 63.26, 61.21, 56.14, 4.95 ,39.30,39.09,37.97,36.60,36.19,35.85,35.38,34.16,33.76,31.50,31.40,29.23,29.10,28.93,28.75,27.85,27.57,24.78,24.60,23.84,23.50,22.28...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com