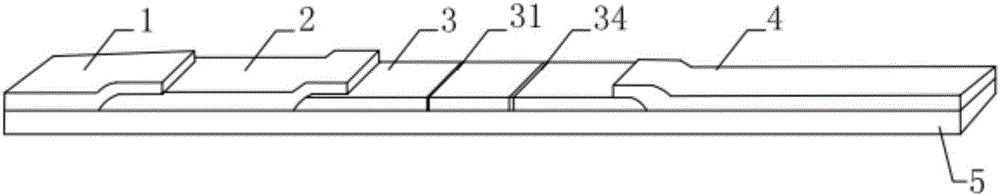
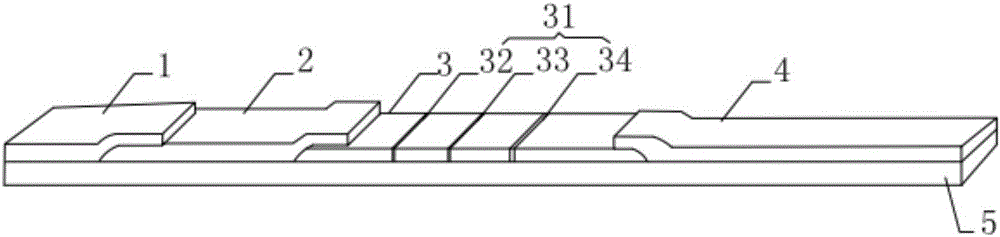
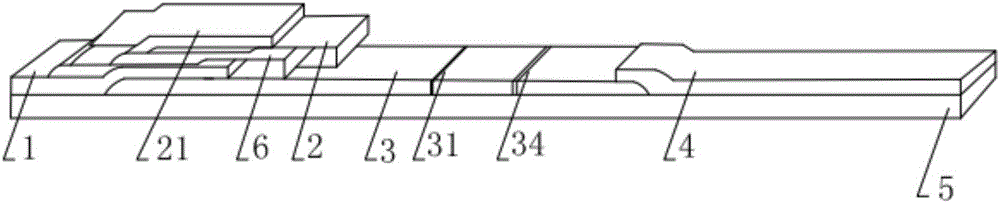
Hyperglycosylated-human chorionic gonadotrophin (H-HCG) rapid immune diagnosis chromatography test paper and preparation method thereof
A chromatographic test strip and rapid immunization technology, applied in the field of medical testing, can solve the problems of limited test results and high false positive rate, and achieve the effects of great application value and clinical significance, high sensitivity, and easy storage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] The preparation method of described a kind of H-HCG rapid immunodiagnosis chromatography test paper comprises the steps:
[0080] The main raw materials are as follows:
[0081] Reagents: chloroauric acid, trisodium citrate, anti-α-HCG or β-HCG antibody, H-HCG specific antibody B207 or B152;, BSA, trehalose, NaCl, Na2HPO4·12H2O, NaH2PO4·2H2O, boric acid, Borax, Tween-20, TritonX-100, sucrose, ultrapure water;
[0082] Consumables: nitrocellulose membrane (NC membrane), 8975 glass fiber, GF-06 glass fiber, absorbent pad, bottom plate, volumetric flask, Erlenmeyer flask, EP tube, pipette tip.
[0083] 1. Preparation before preparation:
[0084] 1.1 Preparation of 2% trisodium citrate solution
[0085] Weigh 0.105g of trisodium citrate into a 15ml EP tube, add 5ml of pure water, and mix until completely dissolved to obtain a 2% trisodium citrate solution.
[0086] Take 40ml of pure water in a 50ml volumetric flask, add 0.5ml of 1% chloroauric acid solution to the volum...
Embodiment 2
[0131] Sensitivity detection of H-HCG rapid immunodiagnostic chromatography test paper
[0132] 1. Preparation of 12.5mIU / ml, 25mIU / ml, 50mIU / ml HCG solution: redissolve HCG standard in PBS solution containing 0.5% BSA, and then prepare 12.5mIU / ml, 25mIU / ml, 50mIU / ml HCG solution.
[0133] 2. Preparation of 110mIU / ml H-HCG solution: redissolve the H-HCG standard in PBS solution containing 0.5% BSA, and then prepare a 110mIU / ml solution.
[0134] 3. Single channel: Put the test strips of the same batch number into 12.5mIU / ml, 25mIU / ml, 50mIU / ml HCG solution and 110mIU / ml H-HCG solution for about 5 seconds, take them out and place them on the table for about 5 seconds. 15 minutes, observe the results, and the test results are valid within 30 minutes. Each test concentration was repeated 3 times, and the test results of each solution were all positive for 3 times before the result of the secondary concentration was judged as positive;
[0135] Dual channel: Put the test strips...
Embodiment 3
[0142] Specific detection of H-HCG rapid immunodiagnostic chromatography test paper
[0143] 1. Solution preparation: 0mIU / ml HCG solution: Weigh 0.5gBSA into a beaker, add 70ml PBS buffer solution to dissolve to 100ml, filter through a 0.22μm filter membrane and set aside.
[0144] 2. 500mIU / ml human luteinizing hormone (hLH) solution: redissolve the hLH standard product with PBS solution containing 0.5% BSA, and prepare it with 0mIU / ml HCG solution to a concentration of 500mIU / ml, which is hLH A solution; use 25mIU / ml HCG solution to prepare it to a concentration of 500mIU / ml, which is hLH B solution; use 110mIU / ml H-HCG solution to prepare it to a concentration of 500mIU / ml, which is hLH C solution.
[0145] 3. 1000mIU / ml human follicle stimulating hormone (hFSH) solution: redissolve the hFSH standard with PBS solution containing 0.5% BSA, and prepare it with 0mIU / ml HCG solution to a concentration of 1000mIU / ml, which is hFSH A solution ; Use 25mIU / ml HCG solution to prepar...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com