Medicine precursor containing long chain fatty acyl group substituted venlafaxine and its prepn and use
A fatty acyl, prodrug technology, applied in the application of diseases, the field of pharmaceutical composition of prodrugs, can solve the problems of increased psychological burden, limitations in the treatment of neuropsychiatric diseases, frequent medication for patients, etc., and achieve excellent long-term effect of effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
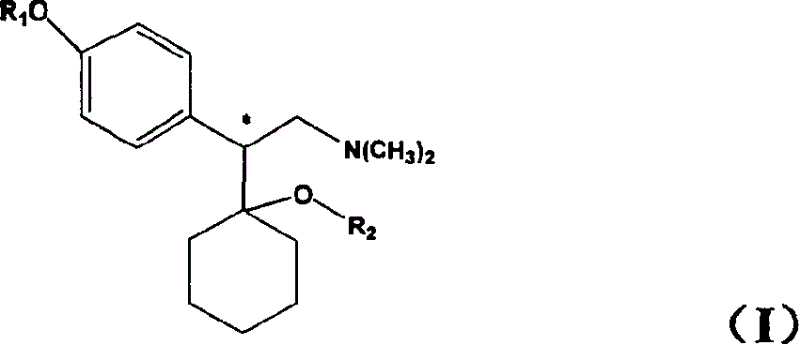
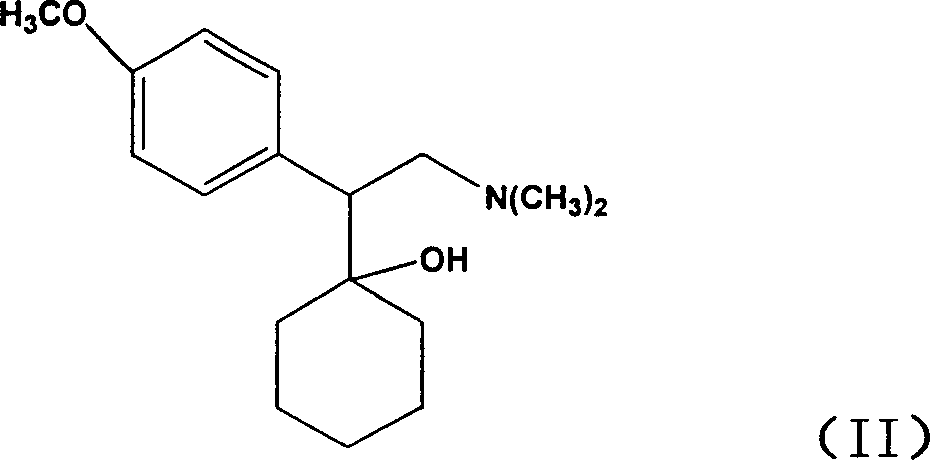
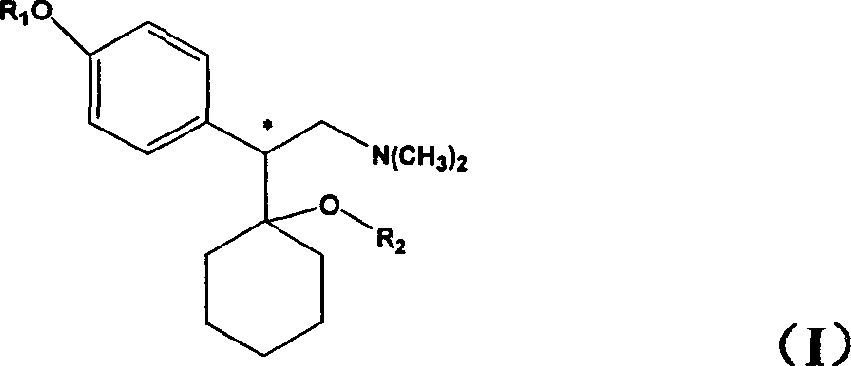
[0023] Chiral venlafaxine can be prepared according to the method described in Yardley et al., J Med Chem, 1990, 33(10): 2899-2905. Optically active enantiomers may also be separated by other resolution techniques routine in the art.
[0024] The pharmaceutically acceptable salt of the compound of the present invention is prepared by direct salt-forming reaction with the free base of the compound and an inorganic or organic acid. The inorganic or organic acid may be selected from hydrochloric acid, hydrobromic acid, phosphoric acid, citric acid, maleic acid and the like.
[0025] The present invention also relates to a pharmaceutical composition prepared by co-preparing a compound as described in general formula I containing a pharmaceutically effective dose and a pharmaceutically acceptable carrier or excipient. Pharmaceutical compositions containing effective doses of the compounds of the present invention can be formulated using pharmaceutical carriers well known to those ...
Embodiment 1
[0035] Embodiment 1: Preparation of octanoic acid 1-[2-(dimethylamino)-1-(4-methoxyphenyl)-ethyl]cyclohexyl ester (B1)
[0036] In a 100ml eggplant-shaped bottle, add 0.50g (1.59mmol) of venlafaxine and 0.46g (3.19mmol) of octanoic acid, dissolve in 15ml of anhydrous dichloromethane, and stir in an ice bath. Add N,N-dicyclohexylcarboimide 0.69g (3.34mmol) and anhydrous dichloromethane 5ml to form a solution, gradually form a white precipitate, ice bath reaction for 2 hours, add N,N-dimethylaminopyridine 0.1 g was used as a catalyst. After removing the ice bath, the mixture was stirred at room temperature for 24 hours. TLC (dichloromethane:methanol 15:1, adding 2 drops of ammonia) showed that the reaction was complete. After the white precipitate N,N-dicyclohexylurea was removed by filtration, the solution was extracted with 5% citric acid aqueous solution (25ml×3), then with saturated sodium chloride aqueous solution (30ml×3), and the dichloromethane solution was added with an...
Embodiment 2
[0040] Embodiment 2: Preparation of nonanoic acid 1-[2-(dimethylamino)-1-(4-methoxyphenyl)-ethyl]cyclohexyl ester (B2)
[0041] The synthesis method is the same as B1. Get 0.50g (1.59mmol) of venlafaxine and 0.56g (3.54mmol) of nonanoic acid to react, the halogenated alkane solvent used is chloroform, and the dehydrating agent is 1-dimethylaminopropyl-3-ethyl carbodiyl imine, the catalyst is 2,4,6-collidine. 0.43 g of white solid (B2) was obtained, with a yield of 64.9%. The hydrochloride was prepared in a similar manner, mp136-138°C. 1 HNMR (CDCl 3 )δ (ppm): 7.16 (d, 2H, J = 8.6Hz, Ph-H), 6.84 (d, 2H, J = 8.6Hz, Ph-H), 3.96-3.93 (dd, 1H, J = 3.1Hz , J'=10.7Hz, CH), 3.78(s, 3H, CH 3 O), 2.96-2.14(tt, 2H, J=3.1Hz, J'=10.7Hz, CH 2 N), 2.26(t, 2H, COCH 2 ), 2.15(s, 6H, N(CH 3 ) 2 ), 1.61-0.86 (m, 25H, cyclohexyl, (CH 2 ) 6 CH 3 ). MS (FAB) m / z: 418.3 (M + ), 260.1 (M + -OCO(CH 2 ) 6 CH 3 ), 58 (CH 2 N(CH 3 ) 2 , base peak).
[0042] Elemental analysis: C H N...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com