Insulinotropic peptide derivative with modified n-terminal charge
a technology of n-terminal charge and peptide, which is applied in the direction of peptide/protein ingredients, extracellular fluid disorder, metabolic disorder, etc., can solve the problems of patients' pain and major inconvenience, and achieve the improvement of insulinotropic activity and in-vivo blood glucose level-lowering activity, and prevent the clearance of insulinotropic peptides.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
n of the Binding Activity of GLP-1 Receptor and the Insulinotropic Peptide Derivative with a Modified N-Terminal Charge
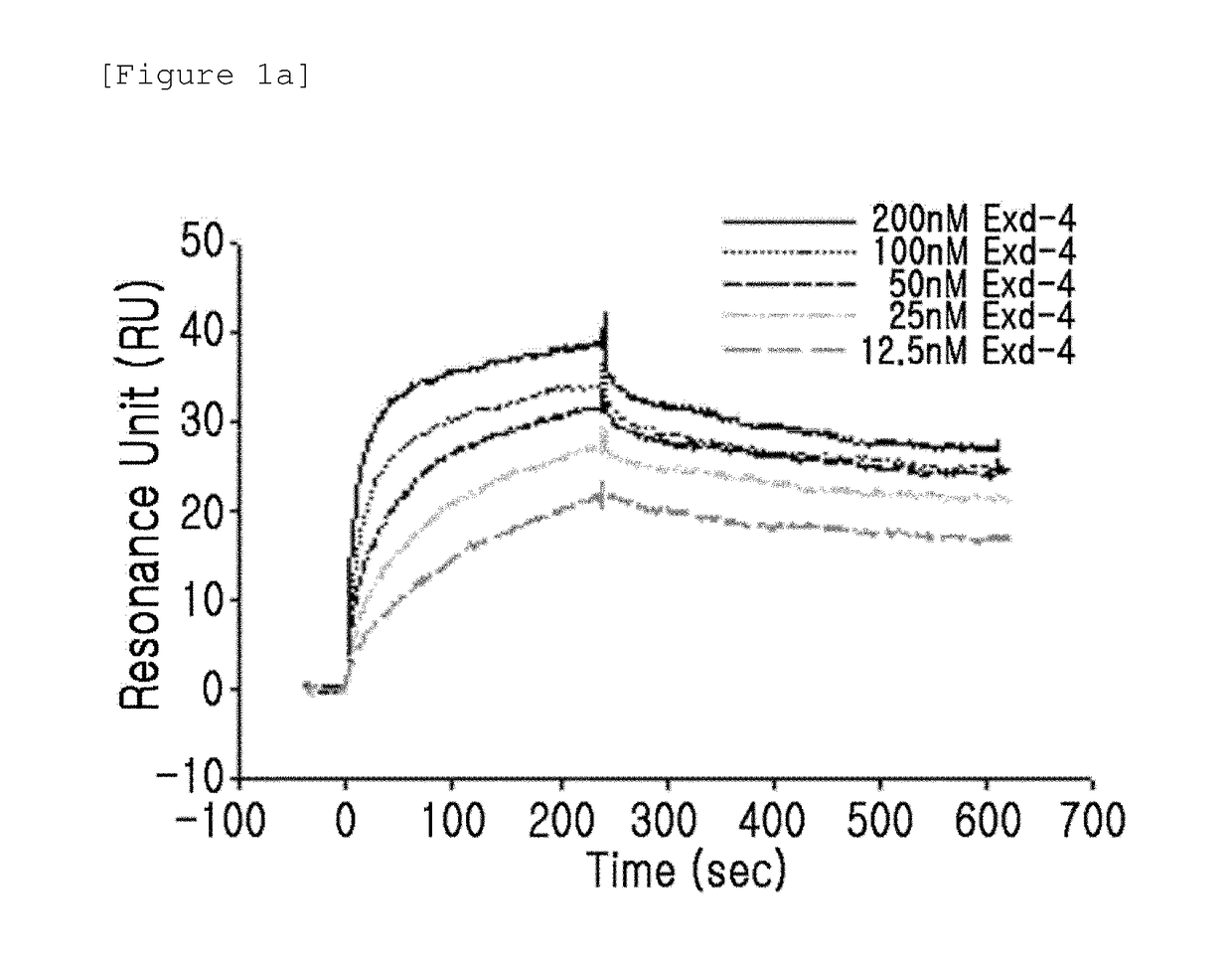
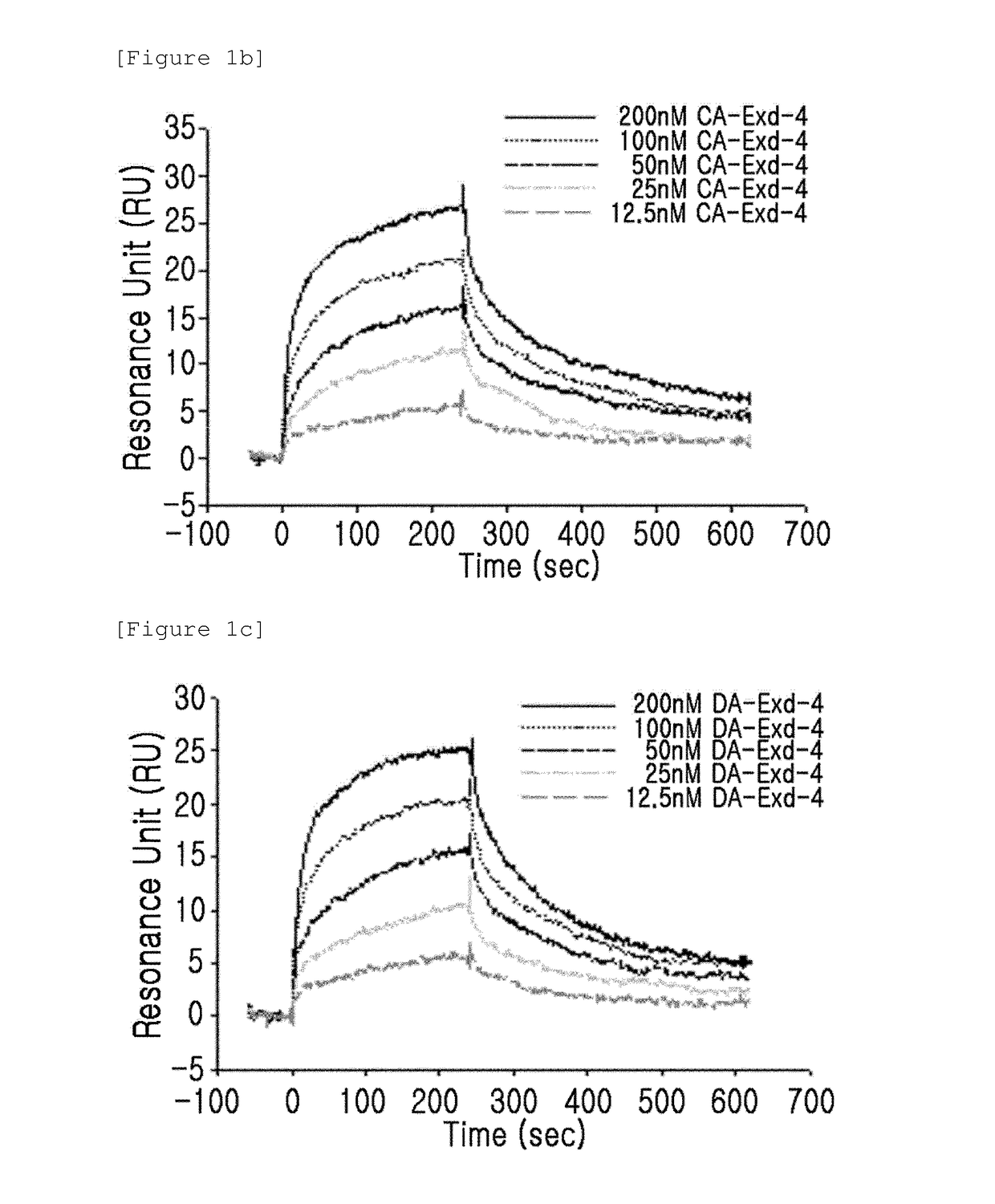
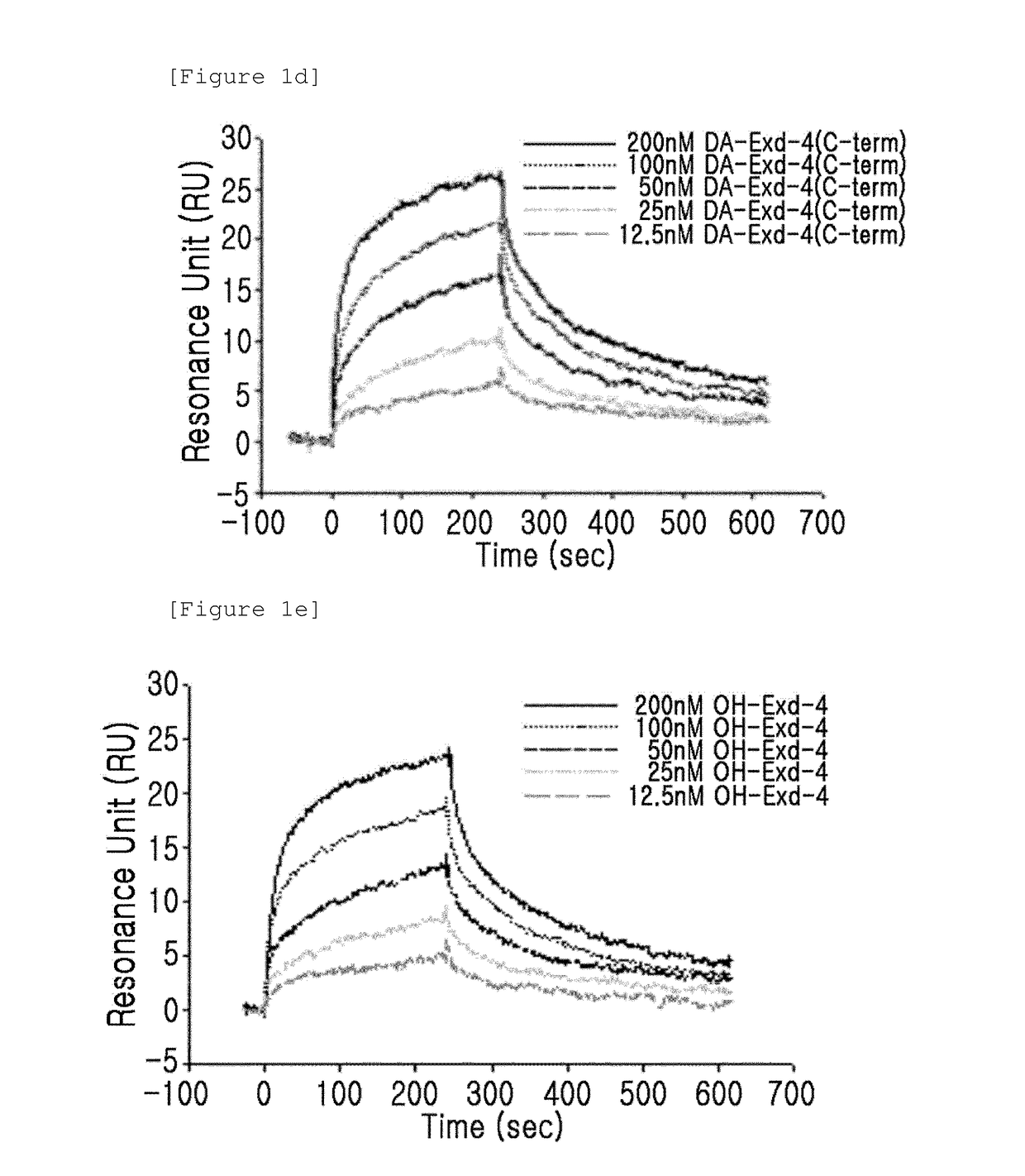
[0089]The binding activity of the GLP-1 receptor and the insulinotropic peptide derivative with a modified N-terminal charge were measured using a surface plasmon resonance (SPR) apparatus (BIACORE 3000, GE Healthcare). In this case, as the insulinotropic peptide derivative with a modified N-terminal charge, CA-exendin-4, DA-exendin-4, HY-exendin-4, and DA-exendin-4-propyl-amide were used.
[0090]CA-exendin-4 is a derivative prepared by removing the alpha-carbon of the N-terminal histidine residue of exendin-4; DA-exendin-4 is a deritivate prepared by removing the N-terminal amino group of exendin-4; HY-exendin-4 is a derivative prepared by substituting the N-terminal amino group of exendin-4 with a hydroxyl group; and DA-exendin-4-propyl-amide is a derivative prepared by removing the N-terminal amino group of exendin-4 while substituting the C-terminal carboxyl group...
example 2
nt of Insulinotropic Activity of the Insulinotropic Peptide Derivative with a Modified N-Terminal Charge
[0094]The insulinotropic activities of the insulinotropic peptide derivatives with a modified N-terminal charge were compared in RINm5F cells. RINm5F cells were thawed, and subcultured at least once, followed by inoculation into a 96-well plate at a density of 1×105 cells / well with a culture medium containing FBS (Gibco, #11082). Then, the cells were cultured in a 5% CO2 incubator at 37° C. for 48 hours. For the measurement of the insulinotropic activities, the culture medium of RINm5F cells was replaced with a fresh medium containing 0.5% FBS, and then incubated for 1 hour.
[0095]Each of the insulinotropic peptide derivatives with a modified N-terminal charge and a native exendin-4 (exenatide: BYETTA®) was diluted with a culture medium containing 0.5% FBS and glucose to yield concentrations from 10 nM to 0.001 nM. At this time, the culture medium not containing exendin-4 was used ...
example 3
n of In-Vivo Efficacy of the Insulinotropic Peptide Derivative with a Modified N-Terminal Charge
[0097]To measure in-vivo efficacy of the insulinotropic peptide derivatives with a modified N-terminal charge, their blood glucose lowering effect was measured in a diabetic animal model, as compared with native exendin-4. The db / db mice (Jackson Lab, 10-12 week-old) were made to fast for 2 hours, and then the insulinotropic peptide derivatives with a modified N-terminal charge and exendin-4 (exenatide: BYETTA®) were administered at an amount of 0.01-1000 mcg / kg via a subcutaneous route, respectively. At this time, a vehicle was similarly administered as a control group, and % change of blood glucose vs. the vehicle was calculated at each concentration. After 1 hour, blood samples were collected from a tail blood vessel to measure blood glucose levels using a glucometer. At each concentration, the ED50 for the blood glucose level-lowering effect vs. the vehicle was calculated using the Pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com