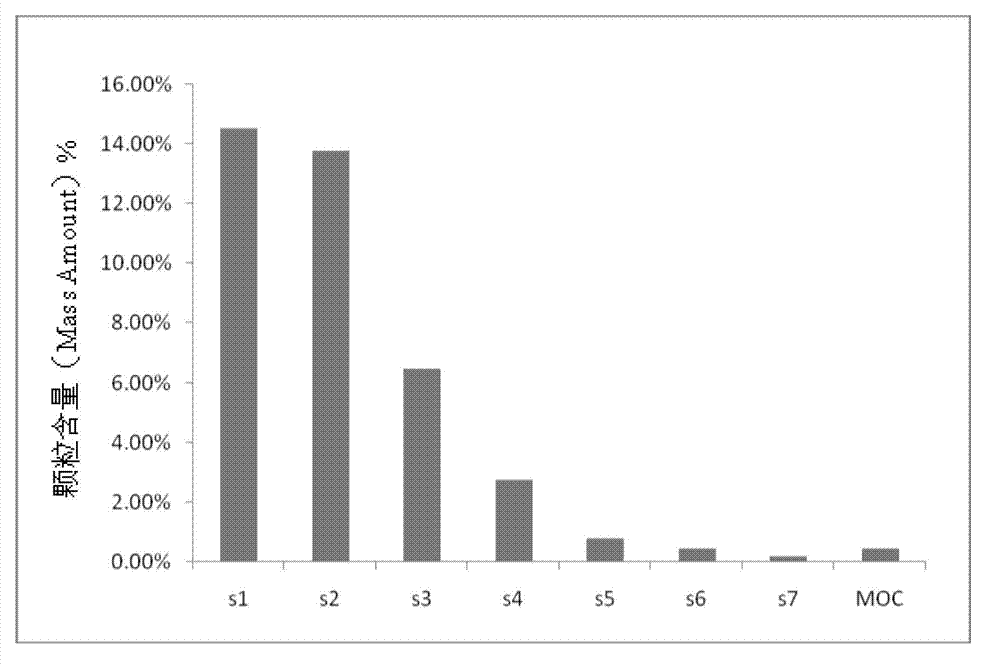
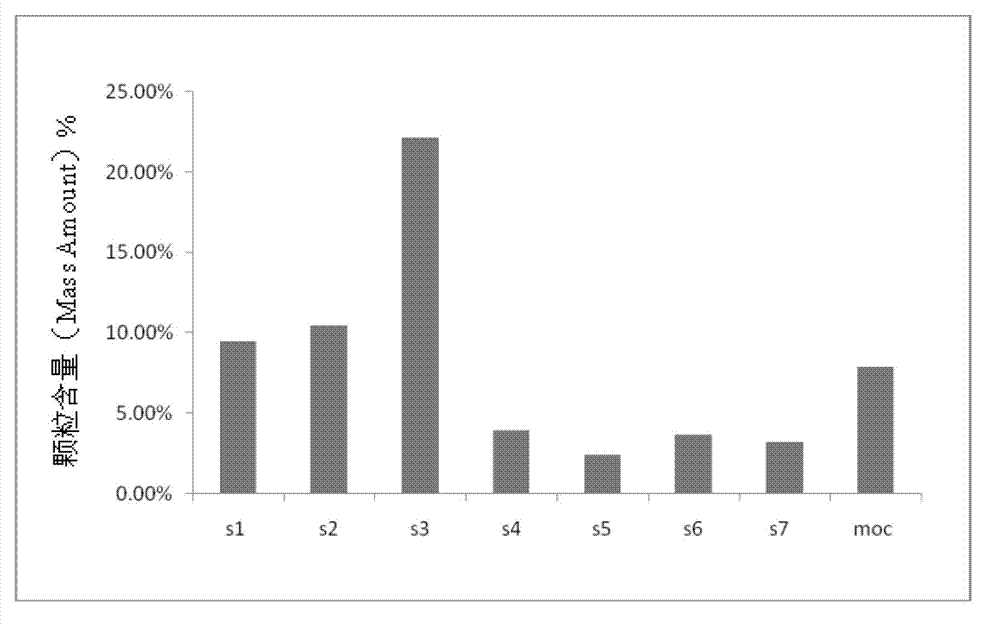
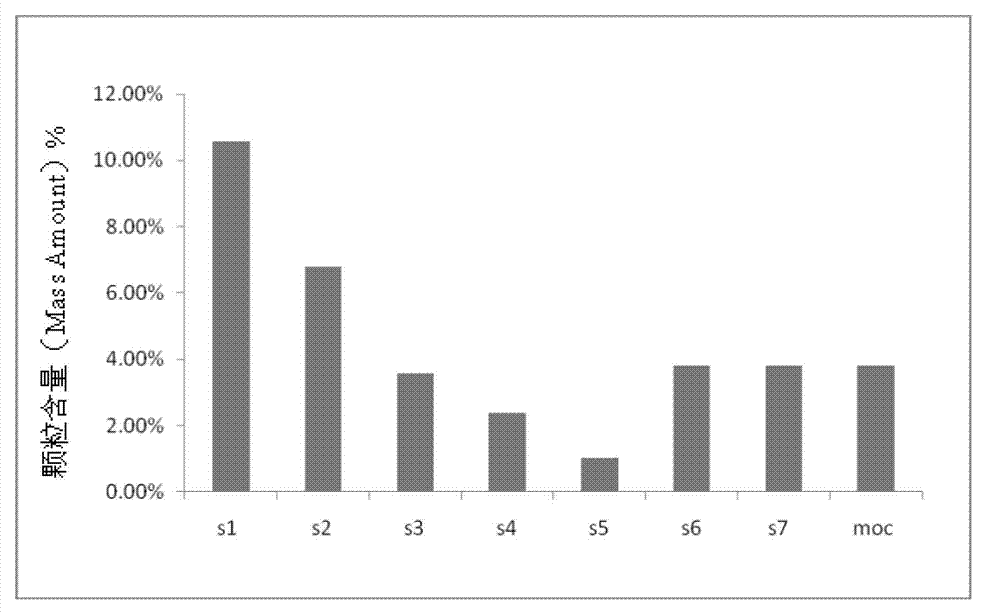
Netilmicin sulfate inhalation powder and preparation method thereof
A technology of netilmicin sulfate and inhalation powder spray, applied in the field of netilmicin sulfate inhalation powder spray and its preparation, can solve the problems of inevitable systemic action, complicated preparation process of injection, poor compliance, etc. Reduced ototoxicity, high effective site deposition, fast onset effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The preparation method of netilmicin sulfate inhalation powder mist of the present invention comprises the following steps: mixing netilmicin sulfate or netilmicin sulfate with amino acid glidants, passing through jet pulverizer, fluidized bed supersonic airflow Grinding in a pulverizer, ball mill, vibrating mill, critical solvent recrystallization or spray drying to obtain a powder with a particle size of 0.1-10 μm, and then fill it into a capsule (preferably No. 3 hydroxypropyl methylcellulose-HPMC) Or in an aluminum blister, ie.
[0051] In the experiment, it was found that the inlet temperature of the spray drying would affect the moisture content of the powder and the particle size of the particles. Reducing the airflow will prolong the time for the droplets to evaporate, and the drying efficiency and yield will also decrease at the same time, because there is less air for evaporating the droplets, and the atomized particles cannot be blown into the cyclone separat...
Embodiment 1
[0059] The raw material composition of netilmicin sulfate inhalation powder mist described in the present embodiment is as follows:
[0060]
[0061] Get 30g of netilmicin sulfate and pulverize it with a micro-jet mill, so that the average particle size of the drug particles is 1-10 μm. After measuring the particle size, pack it into a capsule. The loading capacity of each capsule is about 20mg, containing 20.00mg of netilmicin sulfate.
Embodiment 2
[0063] The raw material composition of netilmicin sulfate inhalation powder mist described in the present embodiment is as follows
[0064]
[0065]
[0066] According to the above formula, take 50g of netilmicin sulfate, dissolve it in deionized water, and prepare a solution of 1670ml. 3 / min, the atomization pressure is 240kpa, and the liquid supply speed is 7ml / min to obtain spray-dried micropowder with a particle size of 1-10μm. After continuing to dry for 15min, collect it in a desiccator for preservation. Determination of drug content, loaded into capsules. The loading capacity of each capsule is about 20mg, containing 20.00mg of netilmicin sulfate.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com