Nasal inhalation powder preparation and device thereof
A powder and preparation technology, applied in the field of pharmaceutical preparations, can solve the problems of poor tissue penetration and low oral bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Precisely weigh an appropriate amount of zanamivir and lactose, place them in a mortar and grind them respectively, pass the ground powder through a 100-mesh and 1250-mesh drying net, and accurately weigh one part of sieved zanamivir and four parts of The sieved lactose is mixed, and after mixing evenly, a single dose of 25 mg is filled in a single-dose nasal inhalation device.
Embodiment 2
[0020] Accurately weigh an appropriate amount of zanamivir, place it in a mortar and grind it, and pass the ground powder through a 100-mesh and 1250-mesh drying net respectively, and accurately weigh one part of sieved zanamivir and four parts of 230 type of lactose, after mixing evenly, fill it in a capsule with a single dose of 25 mg, and place the capsule in a nasal cavity inhalation device during use.
Embodiment 3
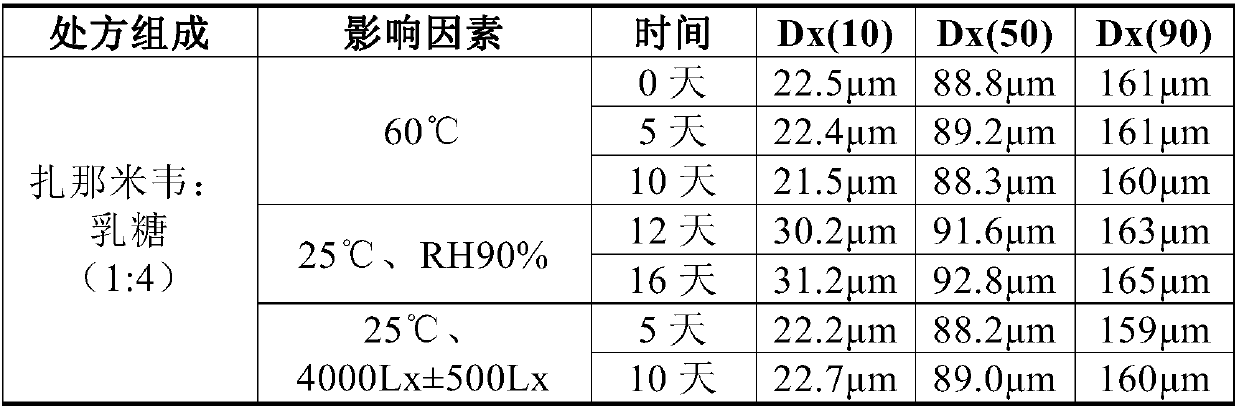
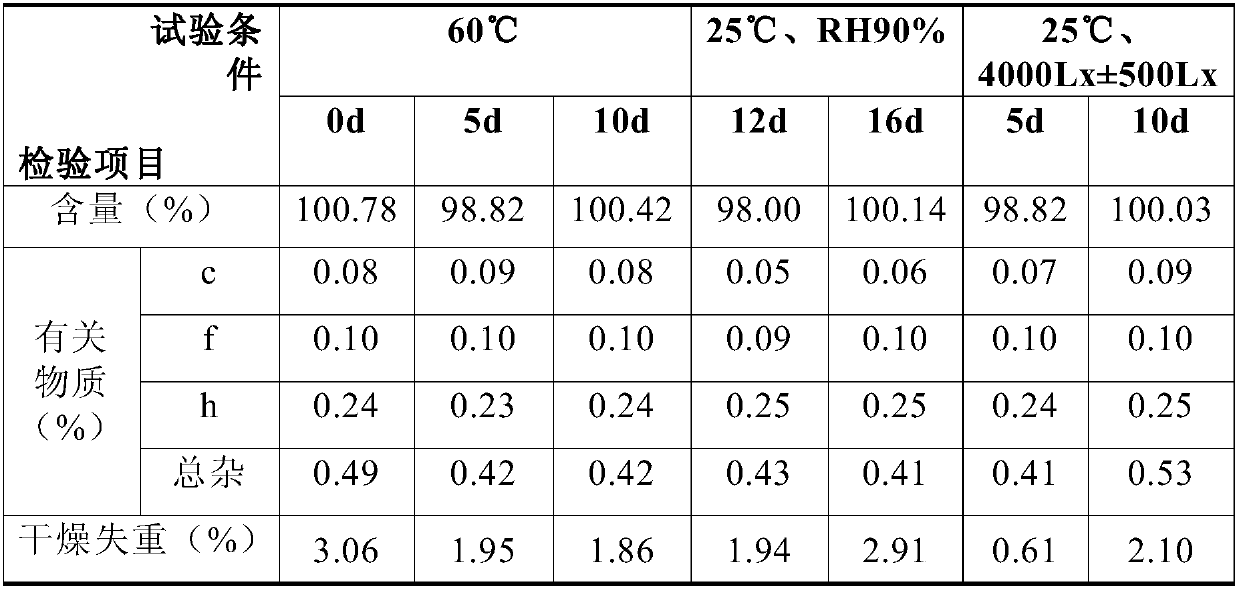
[0022] The Zanamivir nasal inhalation powder formulation constructed in Example 2 is tested, including:
[0023] (1) Particle size of the powder: tested by Malvern laser particle size analyzer Mastersizer 3000, the method is dry test, the parameters are: air pressure 2bar, refractive index 1.52.
[0024] Changes in particle size during the stability process of self-developed samples
[0025]
[0026] The results show that the particle size of the sample does not change significantly under the conditions of the influencing factors, indicating that the dispersion of the sample is relatively stable.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com