Novel inhalation preparation
An inhalation preparation and particle size technology, which is applied to respiratory diseases, medical preparations containing active ingredients, pharmaceutical formulas, etc., can solve the problem of large surface energy, poor physical stability, and difficulty in actively inhaling powder mist, etc. problems, to achieve good stability, high potency or bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
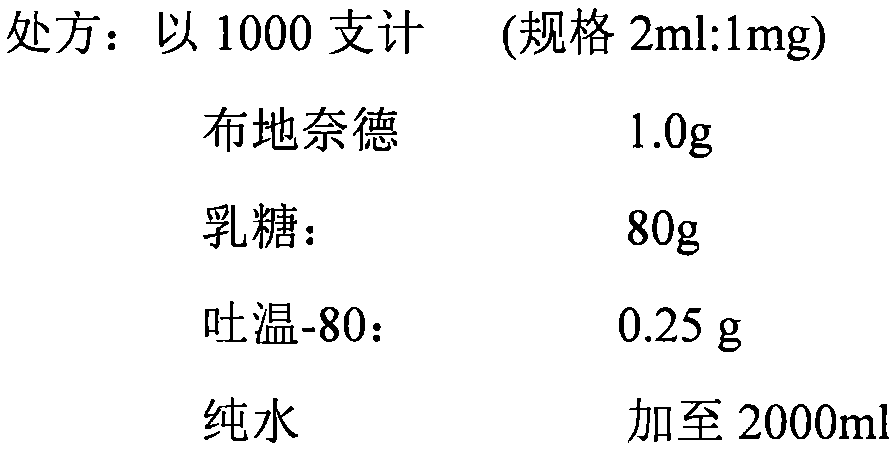
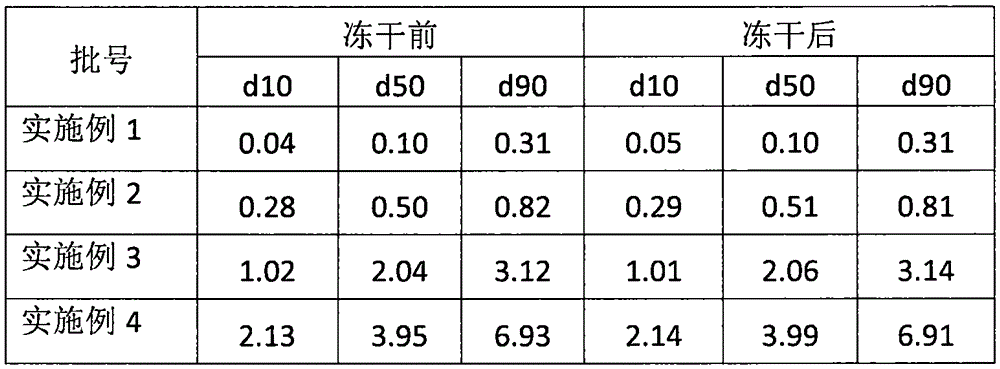
[0014] Example 1 Novel Inhalation Formulation Containing Budesonide
[0015]
[0016] Preparation Process:
[0017] 1. Adopt the wet grinding method, the equipment is DYNO-MILL RESEARCH LAB bead mill, the diameter of the grinding beads is 0.1mm, mix budesonide, auxiliary materials and pure water and add them to the hopper, start the machine, the speed is 1500 rpm, through the type, Grind twice.
[0018] 2. The ground suspension was divided into glass vials for injection, 2ml per vial, and vacuum freeze-dried to obtain solid powder.
[0019] 3. Cover and seal.
Embodiment 2
[0020] Example 2 Novel Inhalation Formulation Containing Budesonide
[0021] Prescription: with embodiment 1
[0022] Preparation process: replace the grinding beads with those with a diameter of 0.5mm, and the rest are the same as in Example 1.
Embodiment 3
[0024] Prescription: with embodiment 1
[0025] Preparation process: replace the grinding beads with those with a diameter of 2.0 mm, and the rest are the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com