A kind of lithium ion battery electrode active material precursor manganese nickel carbonate
A technology for electrode active materials and lithium-ion batteries, which is applied in the direction of battery electrodes, active material electrodes, positive electrodes, etc., can solve the problems of difficult control of shape, uneven particle size, increased uncertainty, etc., and reach the particle size distribution range The effect of small size, uniform particle size and regular shape
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
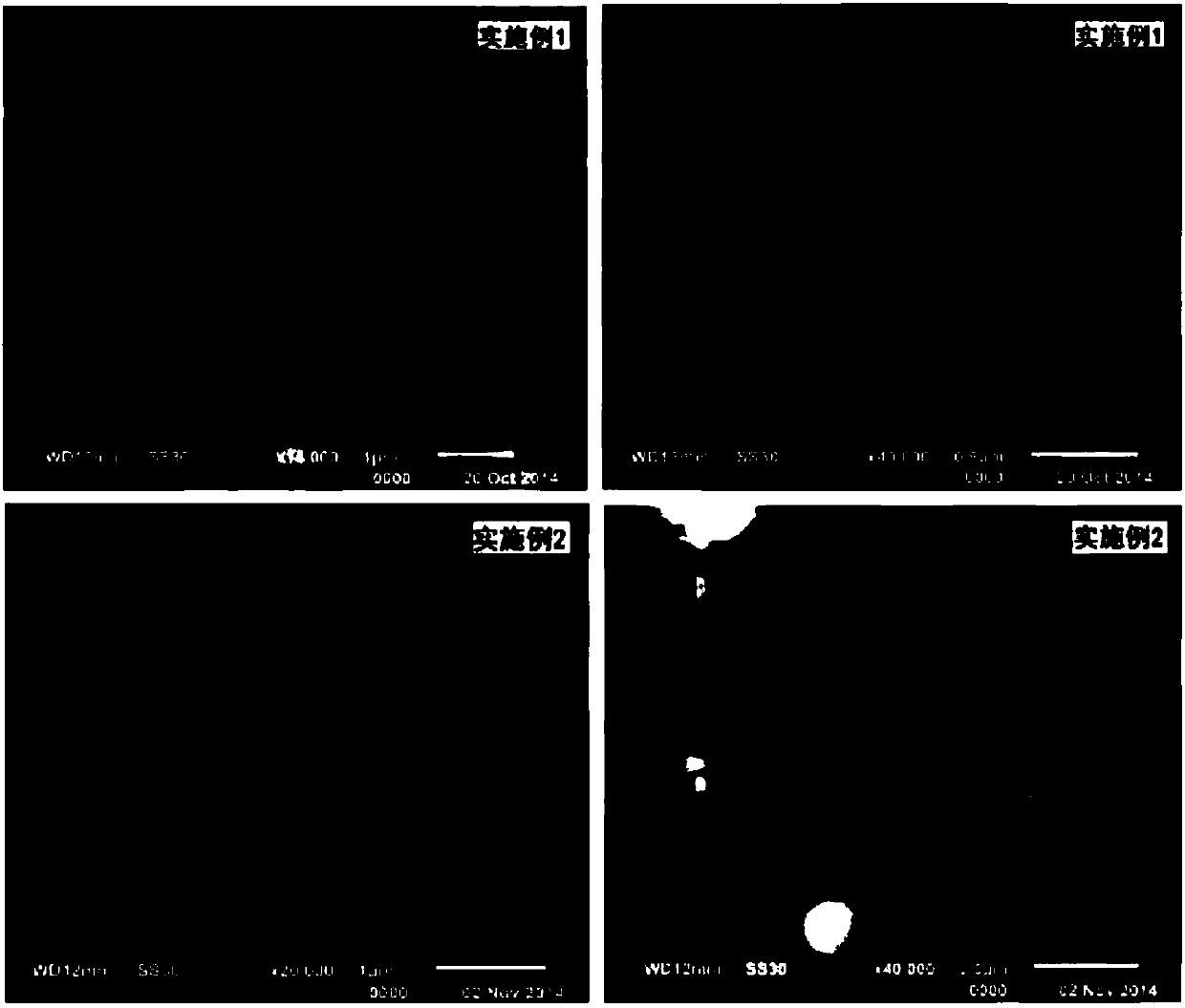
Embodiment 1
[0067] A lithium ion battery electrode active material precursor manganese nickel carbonate, its preparation steps are:
[0068] The first step is to dissolve the mixture of 1.0mmol nickel sulfate and 3.0mmol manganese sulfate with 5mL deionized water to make A solution; the molar weight is 2.2 times the total molar number of the above-mentioned divalent nickel salt and divalent manganese salt nickel manganese , that is, a mixture of 8.8mmol of ammonium bicarbonate precipitant and 12mmol of CTAB surfactant, respectively add 5mL of deionized water, 5mL of n-butanol additive and 100mL of cyclohexane solvent, and stir until the carbonate precipitant and surface The active agent is completely dissolved, that is, there is no solid precipitation, the solution is transparent or translucent, and it is made into B solution;
[0069] In the second step, in the case of continuously stirring the B solution, inject the A solution into the B solution at a uniform speed within 1 minute, and ...
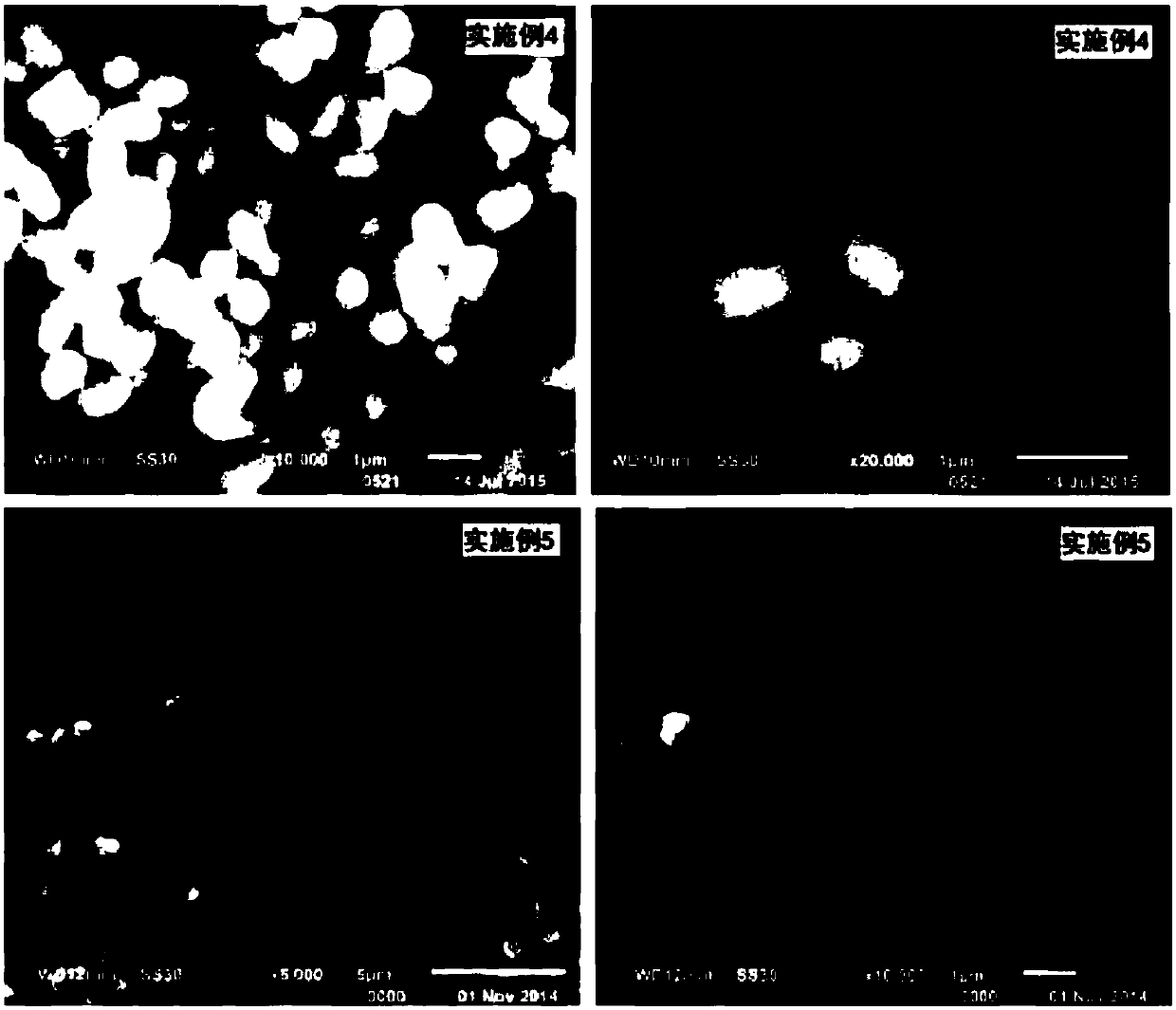
Embodiment 2
[0073] A lithium ion battery electrode active material precursor manganese nickel carbonate, its preparation steps are:
[0074] The first step is to dissolve the mixture of 6.0mmol of nickel sulfate and 12.0mol of manganese sulfate with 15mL of pure water to make A solution; the molar weight is 2.306 times the total molar amount of the above-mentioned divalent nickel salt and divalent manganese salt nickel manganese , that is, 41.5mmol of sodium bicarbonate precipitant, 40mmol of surfactant CTAB, add 15mL of purified water, 15mL of n-butanol additive and 300mL of cyclohexane solvent respectively, and stir until the carbonate precipitant and surfactant Completely dissolved, that is, no solid precipitation, the solution is transparent or translucent, and it is made into B solution;
[0075] In the second step, in the case of continuously stirring the B solution, within 5 minutes, inject the A solution into the B solution at a constant speed, and continue to stir for 10 minutes;...
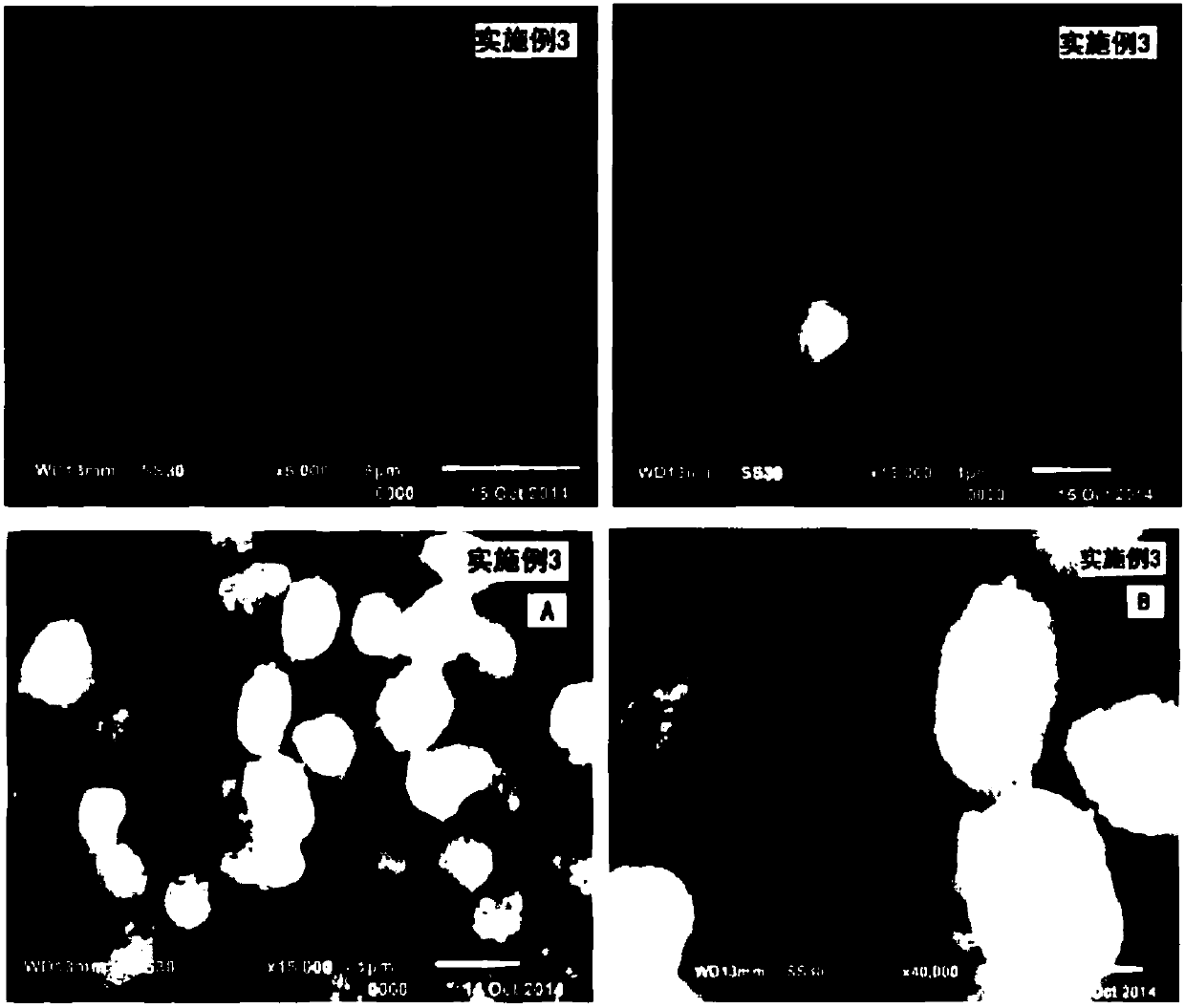
Embodiment 3
[0079] A preparation method of lithium ion battery electrode active material precursor manganese nickel carbonate, the steps are:
[0080] The first step is to dissolve the mixture of 20mmol nickel sulfate and 60mmol manganese sulfate with 40mL distilled water to form a solution; the molar amount is 2.2 times the total molar number of the above-mentioned divalent nickel salt and divalent manganese salt nickel manganese, that is, 0.165mol ammonium bicarbonate precipitant, 120mmol of the CTAB surfactant mixture, respectively, respectively added 40mL of distilled water, 40mL additive n-butanol and 800mL cyclohexane solvent, stirred until the carbonate precipitant and surfactant completely dissolved, that is, no Precipitation, solution transparent or translucent, made into B solution;
[0081] In the second step, in the case of continuously stirring the B solution, within 20 minutes, inject the A solution into the B solution at a uniform speed, and continue to stir for 10 minutes;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com