Capsule type Eletriptan nose inhalation powder aerosols and preparation method and application thereof
A technology of eletriptan and inhalation powder spray, applied in the field of pharmaceutical preparations, can solve the problems of dryness, itching, burning sensation, reduced medication compliance, low bioavailability, etc. Eliminate irritation and discomfort, reduce drug dosage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Recipe: eletriptan 2g, lactose 15g, made into 1000 capsules in total.
[0028] Take 2 g of eletriptan, dry at 50°C for 1.5 hours, and let cool to room temperature; the raw material is micronized with a jet mill; 15 g of lactose is ultrafinely pulverized, passed through a 60-mesh sieve, and the sieved lactose is placed in In a fluidized bed granulator, ethanol is used for granulation, the inlet air temperature is 60°C, the material temperature is 45°C, the liquid spray speed is 2g / min, and the fan frequency is 20Hz. After spraying, continue to dry for 15 minutes.
[0029] Take the micronized eletriptan and lactose granules, and mix them in equal amounts. The mixing speed is 5 rpm, and the mixing time is 20 minutes, and the mixed powder is filled into capsules with a microcapsule filling machine.
Embodiment 2
[0031] Recipe: 4g of eletriptan, 25g of lactose, made into 1000 capsules in total.
[0032] Take 4g of eletriptan, dry at 60°C for 1 hour, and let cool to room temperature; the raw material is micronized by a jet mill; 30g of lactose is ultrafinely pulverized, passed through a 80-mesh sieve, and the sieved lactose is placed in In a fluidized bed granulator, ethanol is used for granulation, the air inlet temperature is 70°C, the material temperature is 60°C, the liquid spray speed is 3g / min, and the fan frequency is 30Hz. After spraying, continue to dry for 25 minutes.
[0033] Take the micronized eletriptan and lactose granules, and mix them in equal amounts. The mixing speed is 10 rpm, the mixing time is 30 minutes, and the mixed powder is filled into capsules with a microcapsule filling machine.
Embodiment 3
[0035] Formula: Eletriptan 5g, lactose 40g, made into 1000 capsules in total.
[0036] Take 8g of eletriptan, dry at 60°C for 2 hours, and let cool to room temperature; the raw material is micronized with a jet mill; 60g of lactose is ultrafinely pulverized, passed through a 100-mesh sieve, and the sieved lactose is placed in In the fluidized bed granulator, ethanol is used for granulation, the air inlet temperature is 70°C, the material temperature is 60°C, the liquid spray speed is 4g / min, and the fan frequency is 40Hz. After the spraying is completed, continue to dry for 20 minutes.
[0037] Take the micronized eletriptan and lactose granules, and mix them in equal amounts. The mixing speed is 12 rpm, the mixing time is 40 minutes, and the mixed powder is filled into capsules with a microcapsule filling machine.
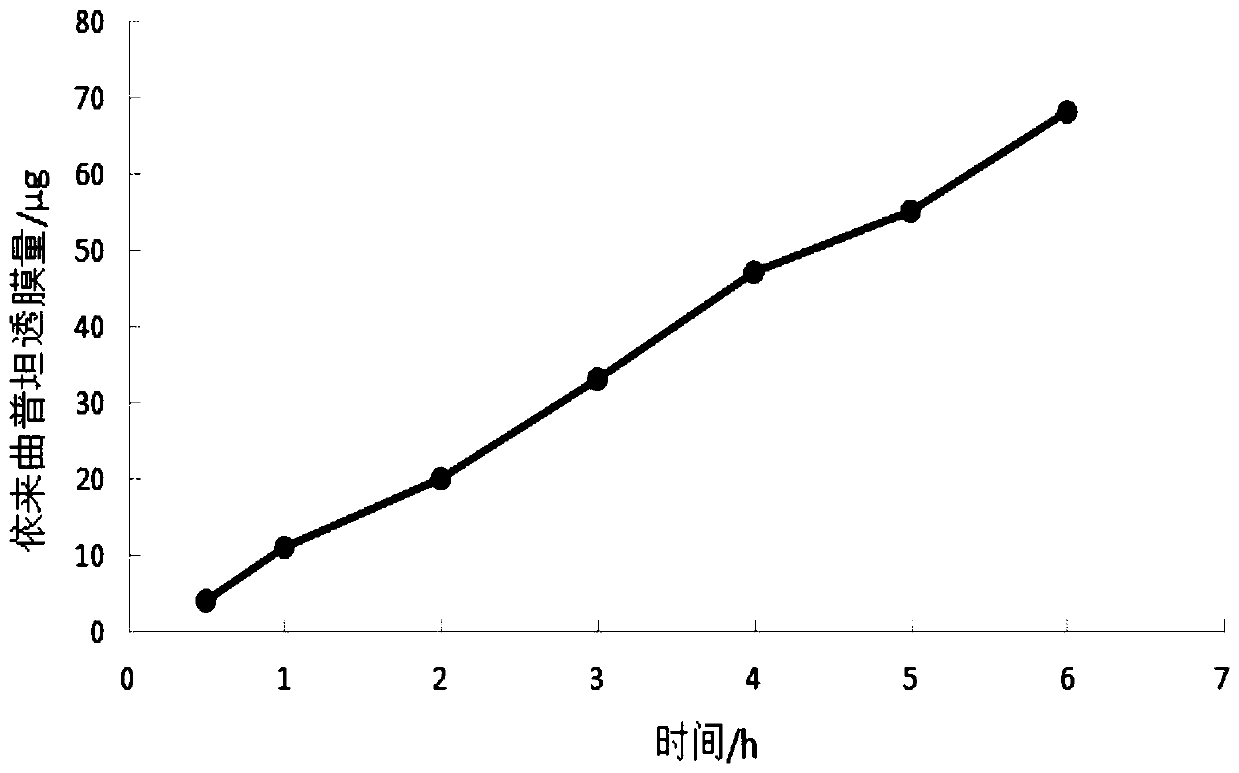
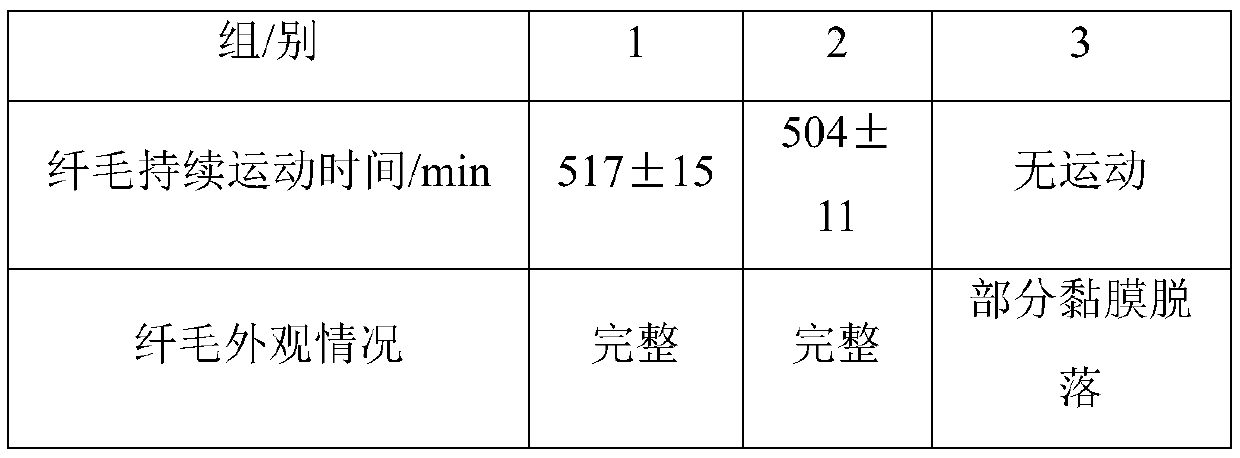
[0038] The present invention has carried out " membrane penetration test " according to following method:
[0039] Method: Take fresh toad mucous membrane, cut ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| D50 | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com