Insulin intranasal inhalation powder spray
A technique for inhaling powder and aerosol insulin, which is applied in the field of nasal insulin preparations, can solve the problems of poor physical stability, achieve rapid onset of action, reduce the risk of nasal irritation, and have high bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
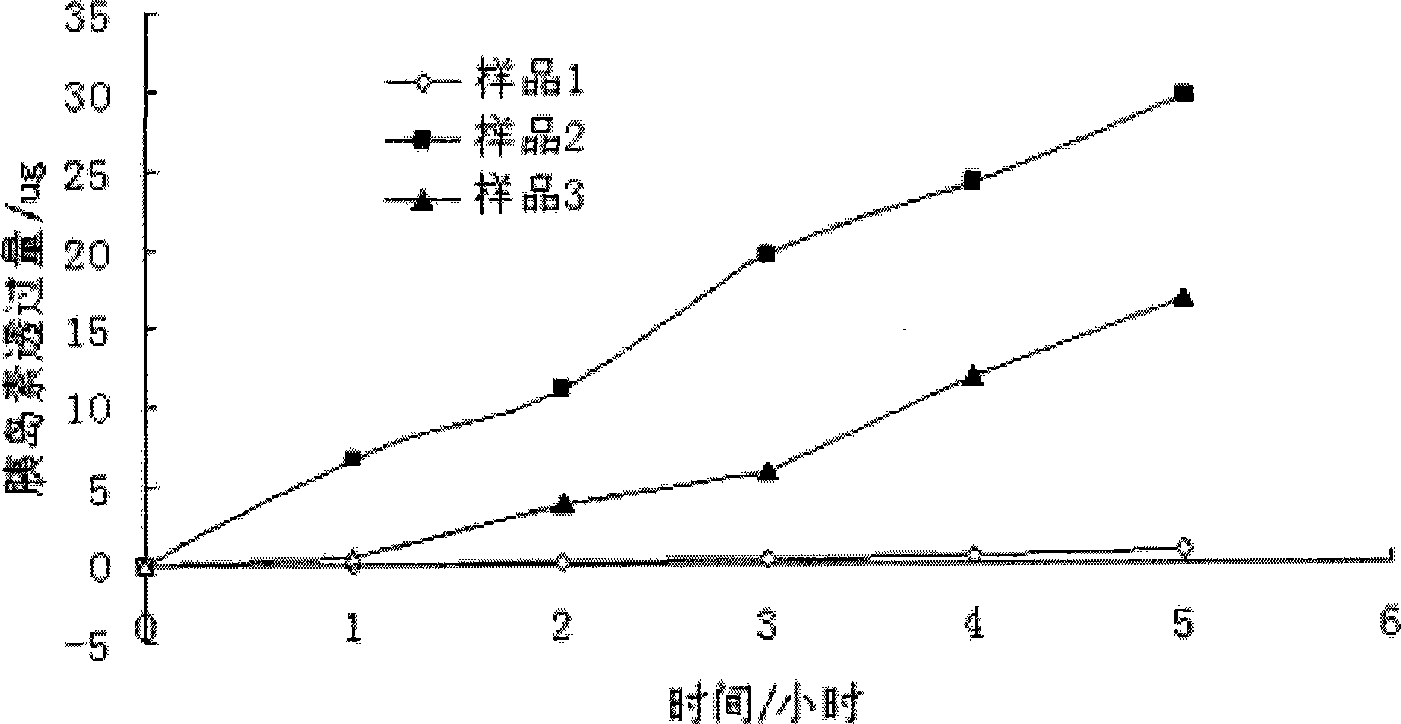
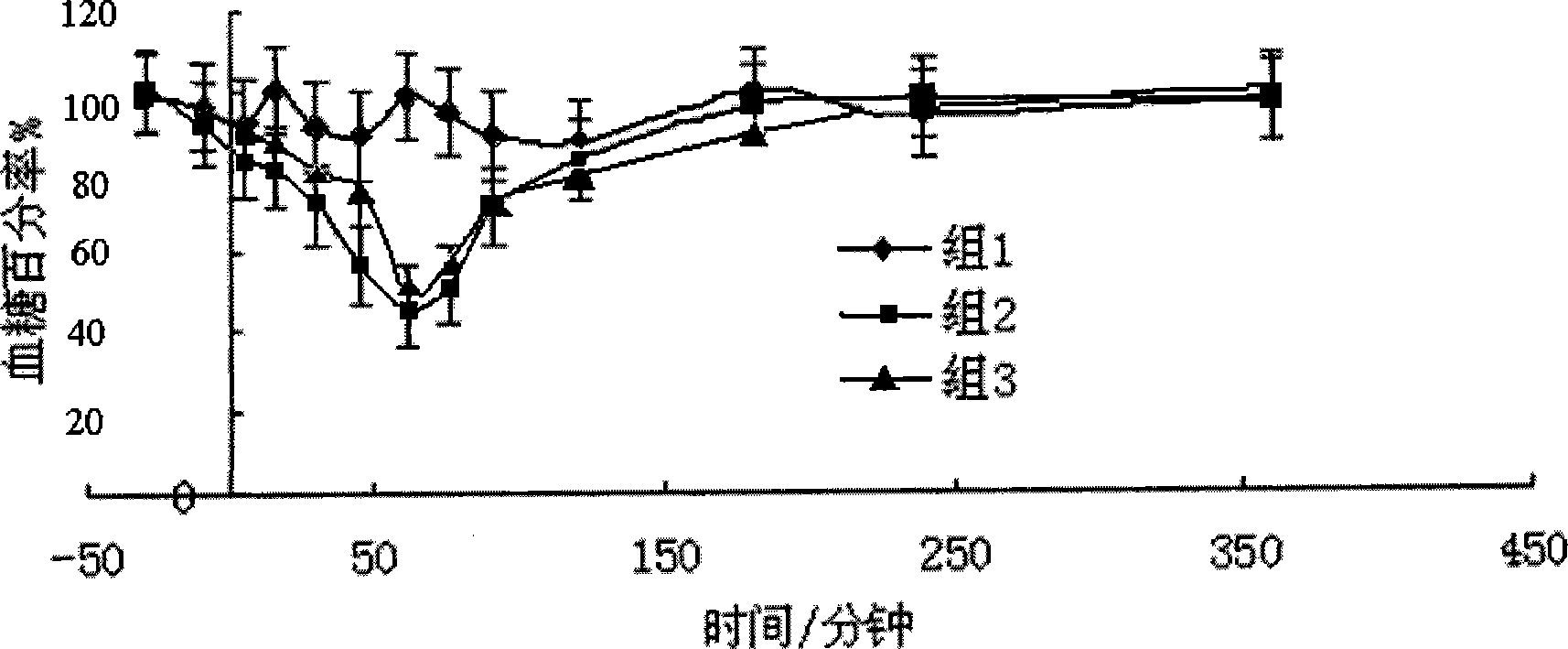
Image
Examples
Embodiment 1
[0093] Get 75mg of chitosan and dissolve it with 1ml0.1mol / l hydrochloric acid for subsequent use;
[0094] Take 1.6g of lecithin, 2.0ml of caprylic acid glyceride, 0.4g of tween80 and 40mg of vitamin E, dissolve at 60°C to obtain the oil phase;
[0095] Pour the oil phase into distilled water at the same temperature at 60°C, stir to obtain colostrum, and circulate the colostrum under a high-pressure milk homogenizer at a pressure of 800 bar for 6 times to obtain 60ml of blank microemulsion. The average particle size of oil droplets is About 45nm;
[0096] Mix 3.0 g of insulin with the above microemulsion, add chitosan solution, add 0.3 ml of 3mol / l HCl dropwise to dissolve insulin; add 3.0 g of mannitol, adjust pH to 4.0 with 0.1 mol / l NaOH, and freeze-dry to obtain insulin nanoemulsion dry powder, freeze-drying procedure:
[0097] -40°C 4h, -15°C 12h, -5°C 4h, 5°C 4h, 20°C 2h, the lowest eutectic point is -9.4°C; the dry powder is sieved and mixed with the mixture of manni...
Embodiment 2
[0099] Get chitosan 75mg and dissolve it with 1ml 0.1mol / l hydrochloric acid for subsequent use;
[0100] Take 1.6g of lecithin, 2.0ml of caprylic acid glyceride, 0.4g of tween80 and 40mg of vitamin E, dissolve at 60°C to obtain the oil phase;
[0101] Pour the oil phase into distilled water at the same temperature at 60°C, stir to obtain colostrum, and circulate the colostrum under a high-pressure homogenizer at a pressure of 600 bar for 6 times to obtain 60ml of blank microemulsion. The average particle size of oil droplets is About 60nm;
[0102] Take 3.0g of insulin and mix it with the above microemulsion, add chitosan solution, add dropwise 0.3ml 3mol / l HCl to dissolve insulin; add 3.0g mannitol, adjust the pH to 4.0 with 0.1mol / l NaOH, freeze-dry to obtain insulin nano Dry powder of milk, freeze-drying procedure: -40°C 4h, -15°C 12h, -5°C 4h, 5°C 4h, 20°C 2h, the lowest eutectic point is -9.6°C; Crystalline cellulose mixed, mannitol:microcrystalline cellulose=1:1, weig...
Embodiment 3
[0104] Get chitosan 75mg and dissolve it with 1ml 0.1mol / l hydrochloric acid for subsequent use;
[0105] Take 1.6g of lecithin, 2.0ml of caprylic acid glyceride, 0.4g of tween80 and 40mg of vitamin E, dissolve at 60°C to obtain the oil phase;
[0106] Pour the oil phase into distilled water at the same temperature at 60°C, stir to obtain colostrum, and circulate the colostrum under a high-pressure homogenizer at a pressure of 400 bar for 6 times to obtain 60ml of blank microemulsion. The average particle size of oil droplets is About 95nm;
[0107] Take 3.0g of insulin and mix it with the above microemulsion, add chitosan solution, add dropwise 0.3ml 3mol / l HCl to dissolve insulin; add 3.0g mannitol, adjust the pH to 4.0 with 0.1mol / l NaOH, freeze-dry to obtain insulin nano Dry powder of milk, freeze-drying procedure: -40°C 4h, -15°C 12h, -5°C 4h, 5°C 4h, 20°C 2h, the lowest eutectic point is -9.8°C; Crystalline cellulose mixed, mannitol:microcrystalline cellulose=1:1, weig...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com