Quality control method for insulin inhalation powder
A quality control method and technology for inhaling powder aerosols, which can be used in aerosol delivery, medical preparations containing active ingredients, and measuring devices, etc., and can solve problems such as the inability to effectively control the quality of insulin inhalation powder aerosols.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: a kind of quality control method of insulin inhalation powder mist is:
[0083] traits:
[0084] The product content is white or off-white powder or granule.
[0085] Identification:
[0086] High-performance liquid chromatography: In the chromatogram recorded under the potency determination item, the retention time of the main peak of the test product should be consistent with the retention time of the peak of the recombinant human insulin reference product. Blank excipients do not interfere with the determination.
[0087] an examination:
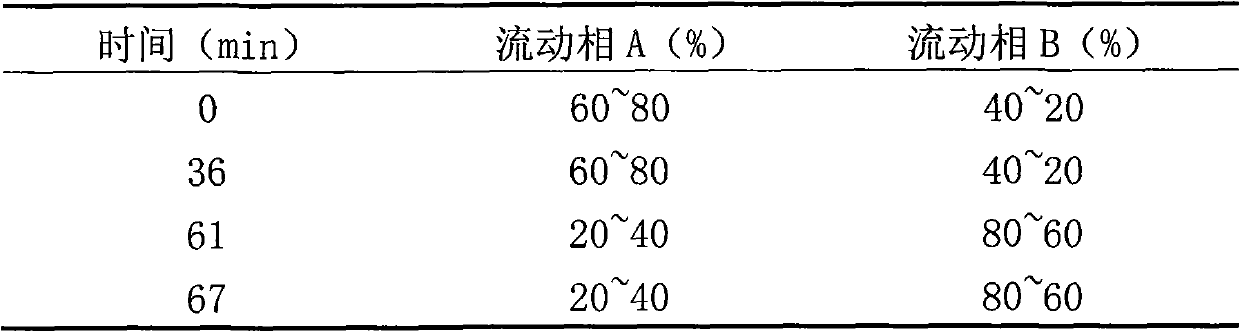
[0088] Related protein inspection: take an appropriate amount of this product, and use 0.01mol / L hydrochloric acid solution to make a solution containing 3mg per 1ml, as the test solution. According to the method under titer determination, use 0.2mol / L sulfate buffer (pH2.3)-acetonitrile (81:19) as mobile phase A, acetonitrile-water (50:50) as mobile phase B, carry out gradient elute.
[0089]
[0090] Adjust...
Embodiment 2
[0100] Embodiment 2: a kind of quality control method of insulin inhalation powder mist is:
[0101] traits:
[0102] The product content is white or off-white powder or granule.
[0103] Identification:
[0104] High-performance liquid chromatography: In the chromatogram recorded under the potency determination item, the retention time of the main peak of the test product should be consistent with the retention time of the peak of the recombinant human insulin reference product. Blank excipients do not interfere with the determination.
[0105] an examination:
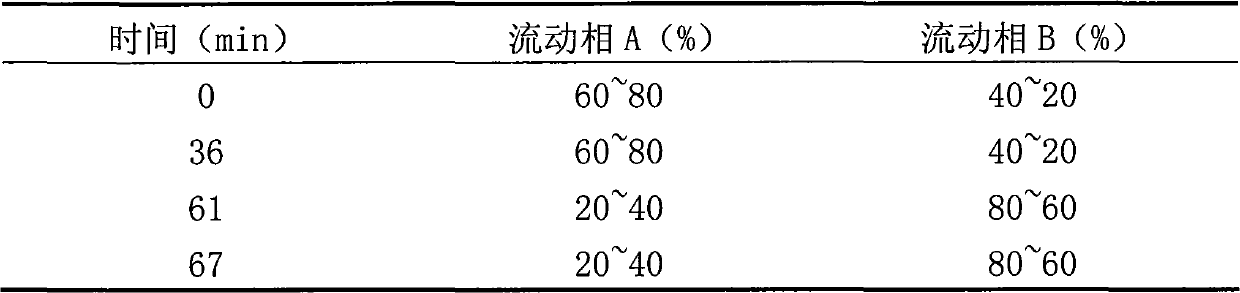
[0106] Related protein inspection: take an appropriate amount of this product, and use 0.01mol / L hydrochloric acid solution to make a solution containing 4mg per 1ml, as the test solution. According to the method under titer determination, use 0.2mol / L sulfate buffer (pH2.3)-acetonitrile (81:19) as mobile phase A, acetonitrile-water (50:50) as mobile phase B, carry out gradient elute.
[0107]
[0108] Adjust...
Embodiment 3
[0118] Embodiment 3: a kind of quality control method of insulin inhalation powder mist is:
[0119] traits:
[0120] The product content is white or off-white powder or granule.
[0121] Identification:
[0122] High-performance liquid chromatography: In the chromatogram recorded under the potency determination item, the retention time of the main peak of the test product should be consistent with the retention time of the peak of the recombinant human insulin reference product. Blank excipients do not interfere with the determination.
[0123] an examination:
[0124] Relevant protein inspection: Take an appropriate amount of this product, and use 0.01mol / L hydrochloric acid solution to make a solution containing 3.5mg per 1ml, as the test solution. According to the method under titer determination, use 0.2mol / L sulfate buffer (pH2.3)-acetonitrile (81:19) as mobile phase A, acetonitrile-water (50:50) as mobile phase B, carry out gradient elute.
[0125]
[0126] Adj...
PUM
| Property | Measurement | Unit |
|---|---|---|
| separation | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com