Naringin nano inhalation powder inhalation taking polylysine as carrier as well as preparation method and application of naringin nano inhalation powder inhalation
A technology for inhaling powder mist and naringin nanotechnology, which is applied in nanotechnology, nanomedicine, nanotechnology, etc., can solve the problems of long inhalation duration, dependence on drug delivery devices, and difficulty in popularization, so as to reduce drug side effects, The effect of improving the curative effect of the drug and the preparation process is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0045]Next, the technical solutions in the embodiments of the present invention will be described in conjunction with the drawings of the present invention. Based on the embodiments of the present invention, all other embodiments obtained by those of ordinary skill in the art are in the range of the present invention without making creative labor premise.
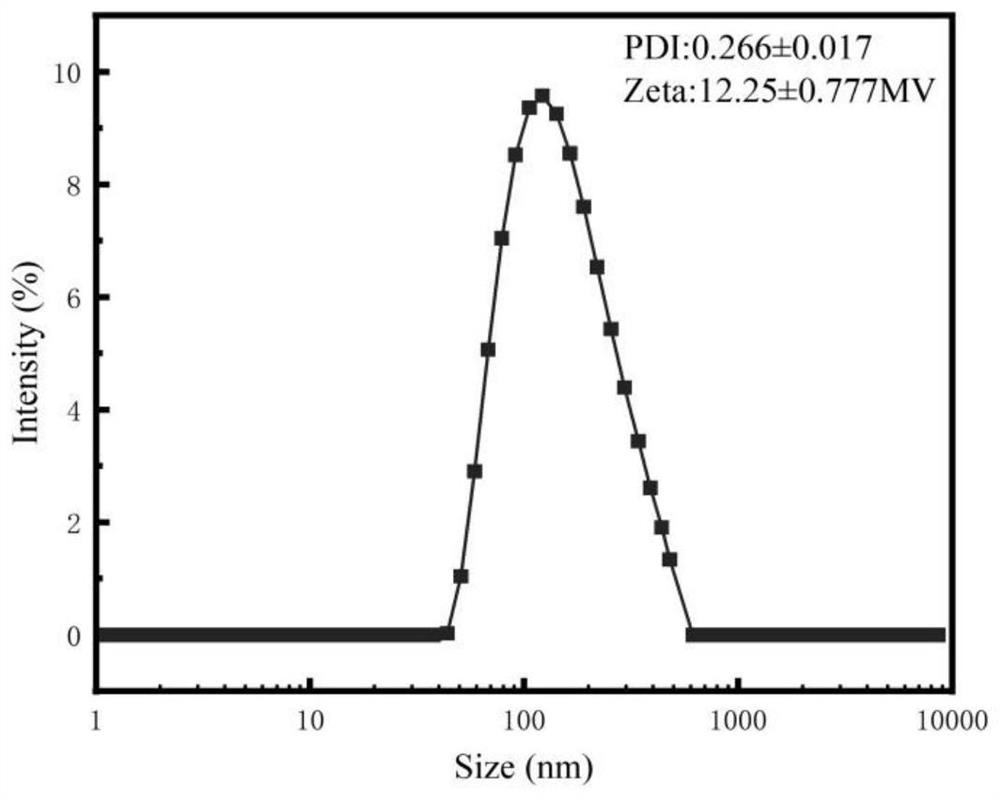
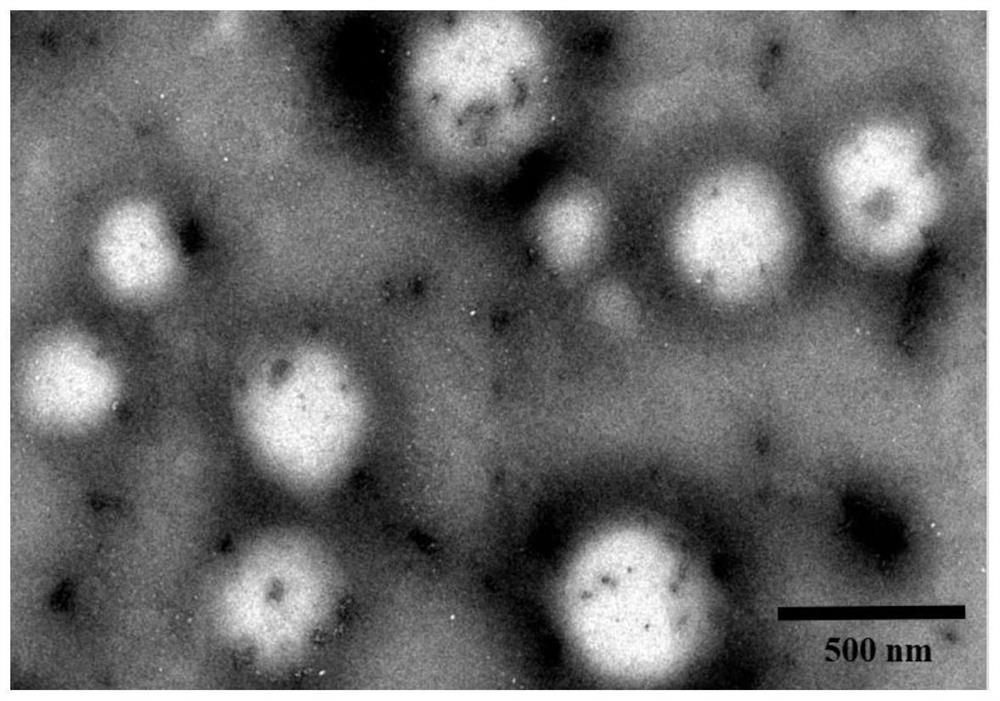
[0046] The example of the present invention discloses a method of preparing a pomardroside nano-induced pocalin nano-induced binder in a vector, not only the preparation process is simple, but also the preparation of teak nano-suspended agent is high, and the accumulated release in vivo Good effect, can improve the efficacy of drugs, suitable for market promotion and application.
[0047] In order to better understand the present invention, the present invention will be further illustrated by the following examples, but it is not understood to be limited to the present invention, and some non-essential improvements and adjustme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com