Chemotherapy-type anticancer drug inhalation powder preparation and preparing method and application thereof
An anticancer drug and powder technology is applied in the field of inhaled dosage forms of inhaled chemotherapy anticancer drugs, which can solve the problems of insufficient active ingredients and strong toxic and side effects, and achieve the effects of reducing high blood pressure, complete absorption, and reducing toxicity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
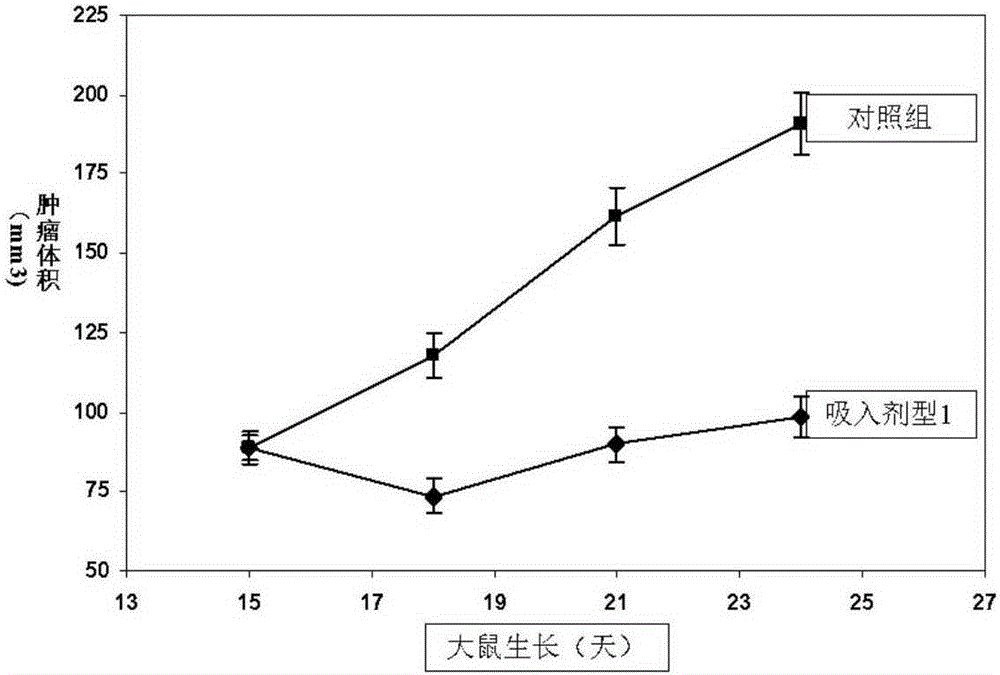
Image
Examples
Embodiment 1
[0037] Example 1 Chemotherapy anticancer drug inhalation powder formulation
[0038] The preparation method is as follows:
[0039] 1) Active ingredient powder
[0040] 2g paclitaxel (or docetaxel, cabazitaxel) was dissolved in 40g absolute ethanol.
[0041] Mix 4.5g of Omega-3 fatty acid sodium salt, 0.4g of phytic acid, 0.1g of poloxamer, 0.1g of vitamin E and 40g of water.
[0042] The above-mentioned paclitaxel solution and Omega-3 solution are thoroughly mixed, the powder is made by freeze-drying method, and the powder is pulverized with a fluid energy mill, and the powder with a particle size of 2-3 μm is selected.
[0043] 2) Carrier powder
[0044] Mix 20 g of lactose, 0.3 g of poloxamer and 1 g of magnesium stearate thoroughly, prepare powder by spray drying method, grind with a fluid energy mill, sieving, and take powder with a particle size of 20-40 μm.
[0045] 3) Mix the above-mentioned active ingredient powder and carrier powder uniformly. This formula is 1,000 unit doses. Ea...
Embodiment 2
[0047] Example 2 Chemotherapy anticancer drug inhalation powder formulation
[0048] The preparation method is as follows:
[0049] 1) Active ingredient powder
[0050] Combine 2g paclitaxel (or docetaxel, cabazitaxel), 4.5g Omega-3 fatty acids (including ethyl ester, or triglycerides, or free acid form), 0.4g phytic acid, 0.4g absolute ethanol, 0.1g poise Loxamer and 0.1 g of vitamin E are thoroughly mixed and dissolved, and the powder is made by spray drying method, and the powder is pulverized with a fluid energy mill, and the powder with a particle size of 2-3 μm is selected.
[0051] 2) Carrier powder
[0052] Mix 20 g of lactose, 0.3 g of poloxamer and 1 g of magnesium stearate thoroughly, prepare powder by spray drying method, grind with a fluid energy mill, sieving, and take powder with a particle size of 20-30 μm.
[0053] 3) Mix the above-mentioned active ingredient powder and carrier powder uniformly. This formula is 1,000 unit doses. Each unit of medicine is packed into a ca...
Embodiment 3
[0055] Example 3 Chemotherapy anticancer drug inhalation powder formulation
[0056] The preparation method is as follows:
[0057] 1) Active ingredient powder
[0058] Mix 2g of paclitaxel (or docetaxel, cabazitaxel), 4.5g of Omega-3 fatty acid sodium salt, 0.4g of phytic acid, 0.4g of absolute ethanol, and 0.1g of vitamin E to dissolve it, and spray-dry it to make a powder. The fluid energy mill grinds to obtain fine powder, and the powder with a particle size of 1-3μm is selected.
[0059] 2) Carrier powder 1
[0060] Mix 2 g of lactose, 0.03 g of poloxamer and 0.1 g of magnesium stearate thoroughly, spray-dry the powder into powder, and grind it with a fluid energy mill to obtain a powder with a particle size of 4-6 μm.
[0061] 3) Carrier powder 2
[0062] Mix 20 g of lactose, 0.3 g of poloxamer, 1 g of magnesium stearate and 1 g of anhydrous ethanol, and use the spray drying method to prepare a powder, which is ground with a fluid energy mill to obtain a powder with a particle size...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com