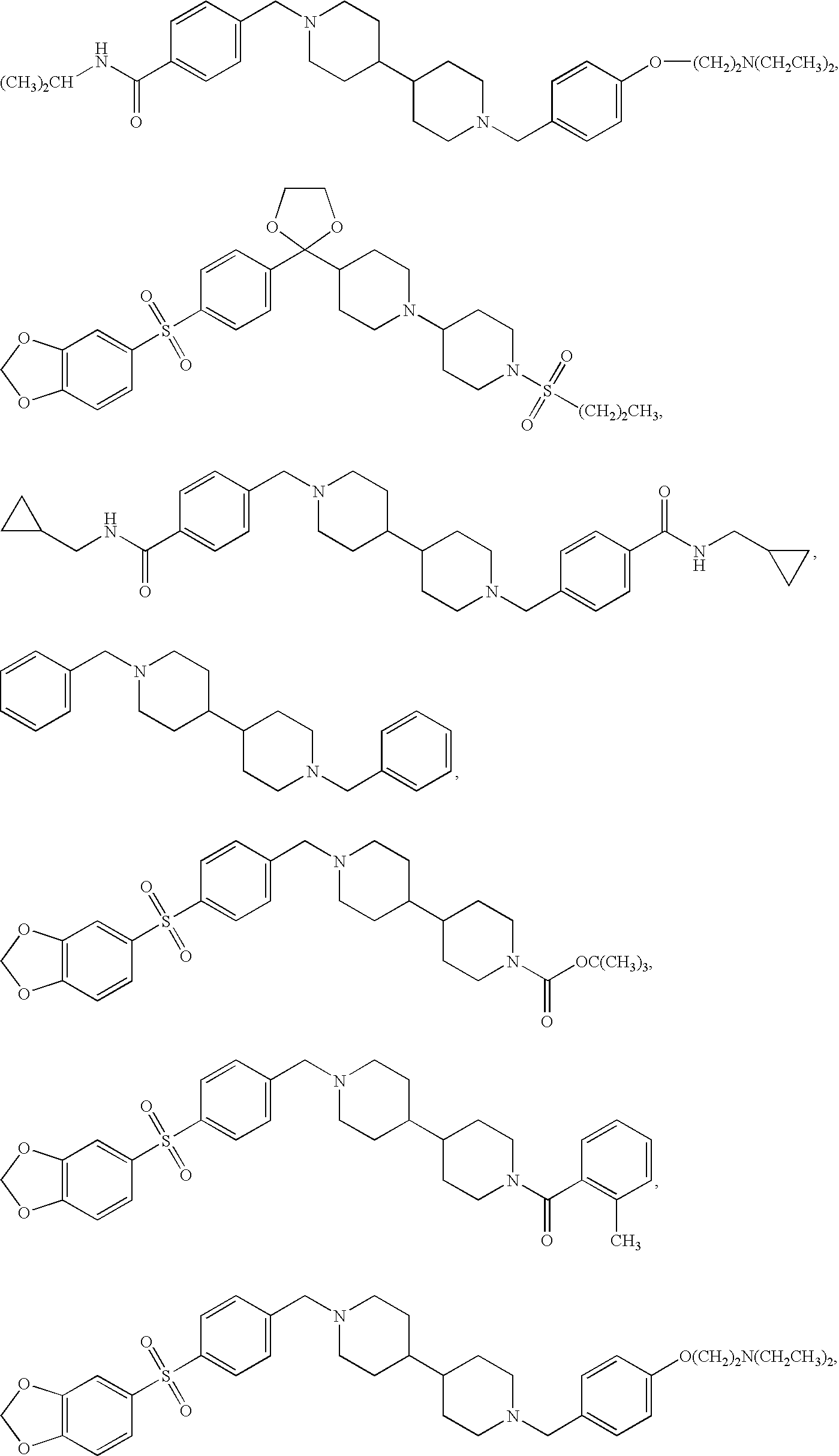
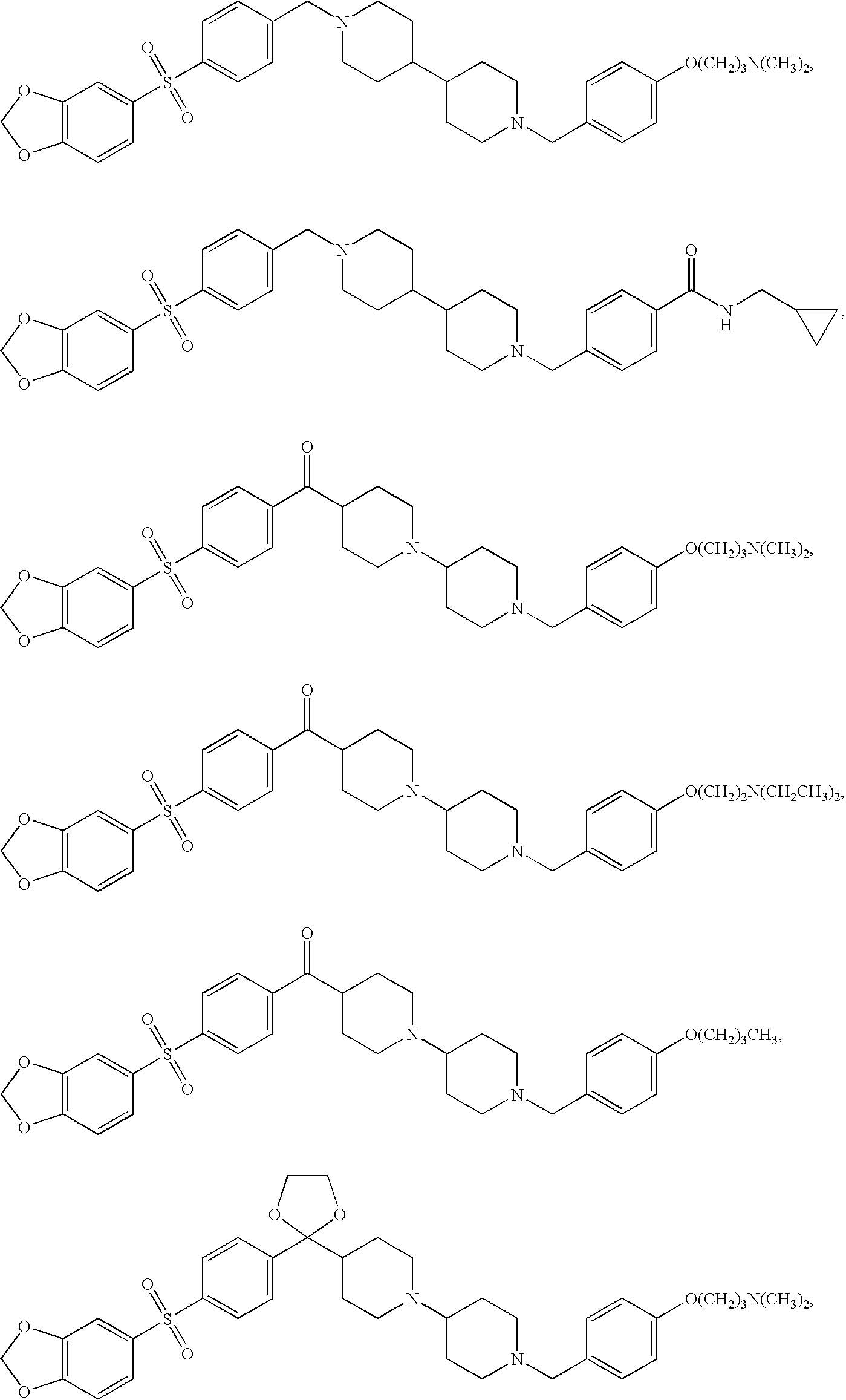
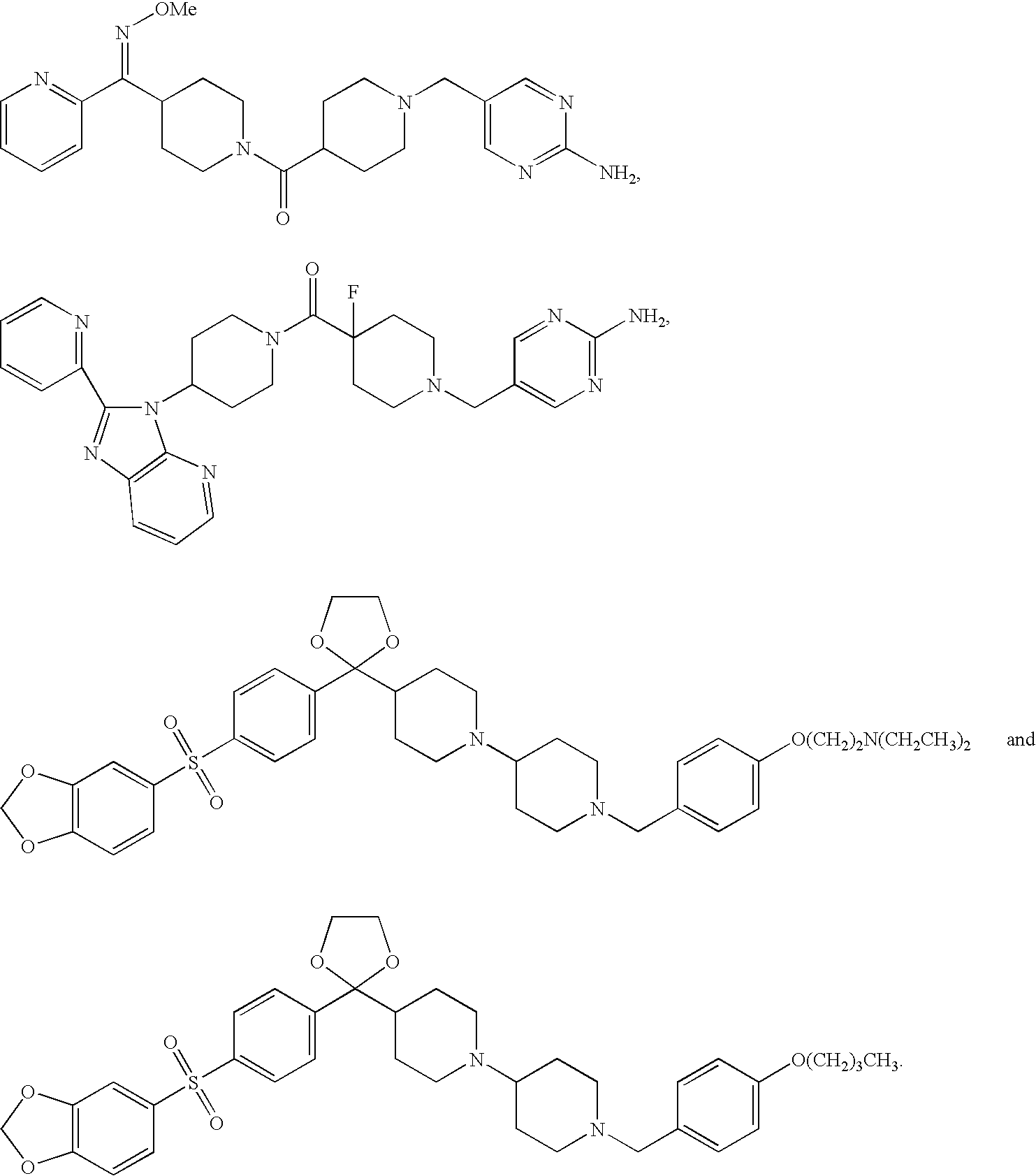
Combination of H1, H3 and H4 receptor antagonists for treatment of allergic and non-allergic pulmonary inflammation, congestion and allergic rhinitis
a technology of histamine receptor and combination, which is applied in the direction of heterocyclic compound active ingredients, drug compositions, biocide, etc., can solve the problems of application that does not disclose the use of histamine h4 receptor antagonists, impaired lung capacity and gas exchange,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Screening Assays for Histamine H4 Receptor Antagonists
[0451] In this example, the ability of several compounds to compete against radiolabeled histamine for binding to membrane-bound human histamine H4 receptor is evaluated. The histamine H4 receptor used in this assay is SP9144 which is set forth in SEQ ID NOs: 1 and 2 of U.S. Pat. No. 6,204,017.
[0452] Membrane preparation. Forty-eight hours after transfection with plasmid containing the sequence for SP9144, HEK293 cells were harvested from T150 flasks by incubating 5 minutes in 5 ml of 5 mM EDTA / Hanks' balanced salt solution followed by repeated pipeting. They were centrifuged 5 minutes at 1000×g. The EDTA / PBS was decanted and an equal volume of ice-cold 50 mM Tris-HCl, pH 7.5, was added and cells were broken up with a Polytron (PT10 tip, setting 5, 30 seconds). Nuclei and unbroken cells were sedimented at 1000×g for 10 minutes and then the supernatant was centrifuged at 50,000×g for 10 minutes. The supernatant was decanted, the...
example 2
Screening Assay for Histamine H1, H3 and H4 Receptor Antagonists
[0460] In the present example, the affinities of several compounds for the H1, H3 and H4 receptors was determined by a membrane binding assay.
[0461] Materials. Rat and guinea-pig brains were obtained frozen from Rockland Immunochemicals (Gilbertsville, Pa.). Cell lines expressing recombinant human receptors were generated by using standard transfection techniques. The following radioligands were obtained from Dupont NEN (Boston, Mass.): [3H]-pyrilamine, 23 Ci / mmol, for H1 binding; [3H]-N′-methylhistamine, 82 Ci / mmol, for H3 binding and [3H]-histamine, 20 Ci / mmol, for H4 binding.
[0462] Methods. Recombinant cell lines (i.e., human H1-CHO cells, human H3-HEK293 and human H4-HEK293 cells) were cultured in Dulbecco's modified Eagle's medium / 10% fetal bovine serum supplemented with 2 mM glutamine, penicillin (100 U / ml), and streptomycin (100 μg / ml) in a humidified 5% CO2 atmosphere at 37° C. Selection was maintained with 0...
example 3
Effect of a Compound Comprising Formula 19 on BAL Cells Recovered from LPS-Challenged Rats
[0467] The following example demonstrates the ability of a compound comprising formula 19 (i.e.,
) to reduce the lipopolysaccharide (LPS)-induced inflammatory response in rat airways.
[0468] Male Sprague-Dawley rats (250-300 g) were anesthetized by inhalation of isoflurane (flow rate 1 ml / min; supplemented with O2). Using a Penn-Centry microspray needle, 0.1 ml of a 100-μg / ml LPS solution in saline was injected into the trachea. Animals not challenged with the LPS solution received 0.1 ml of saline. Animals were placed on a heat pad until they recovered from anesthesia. Afterward, they were returned to their cages and allowed food and water ad libitum. All animals survived these manipulations and no additional interventions were required to ensure their survival. Animals fasted overnight were orally dosed with either the standard PD4 inhibitor and positive-control, SB207499 (c4-cyano-4-(3-cy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Pharmaceutically acceptable | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com