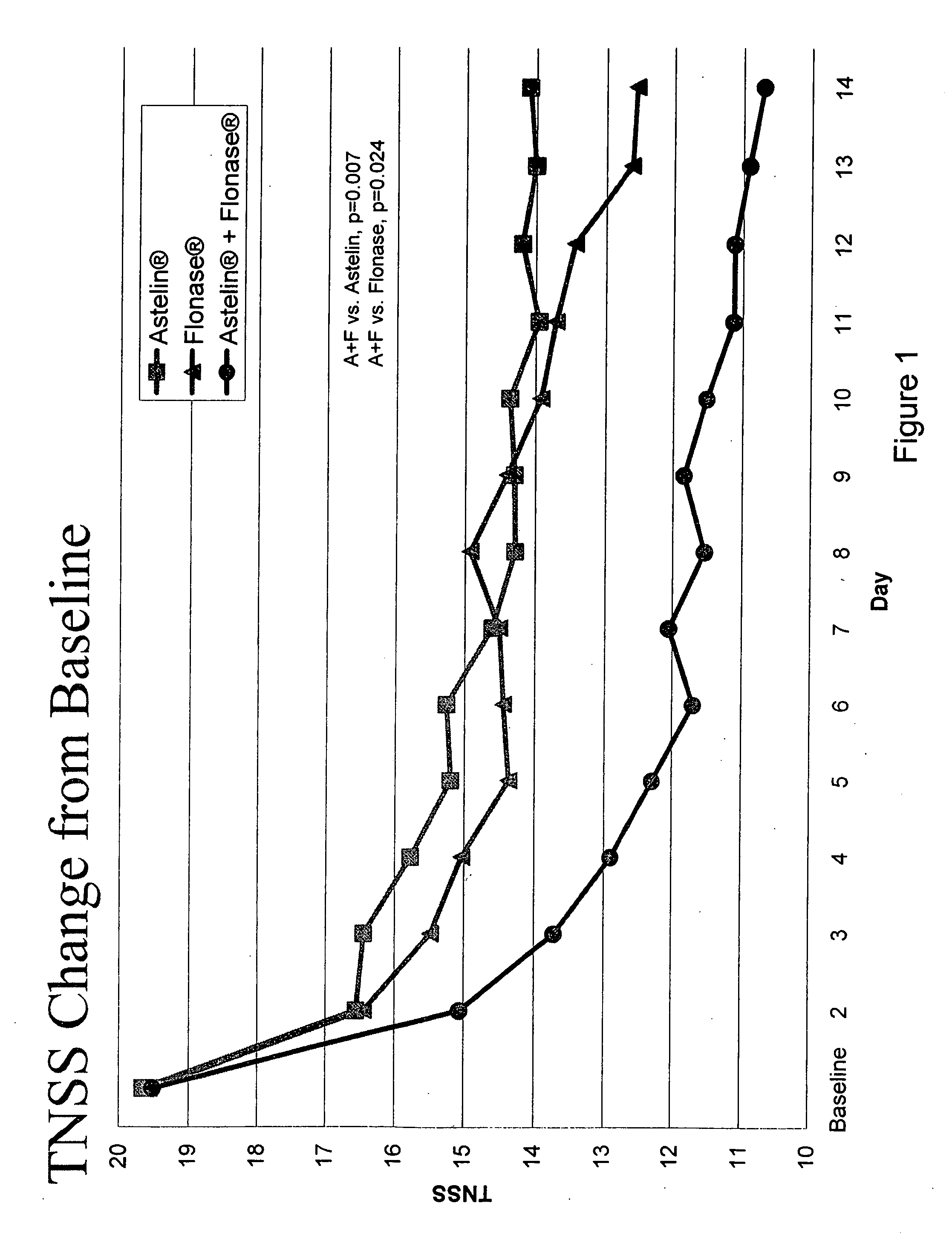
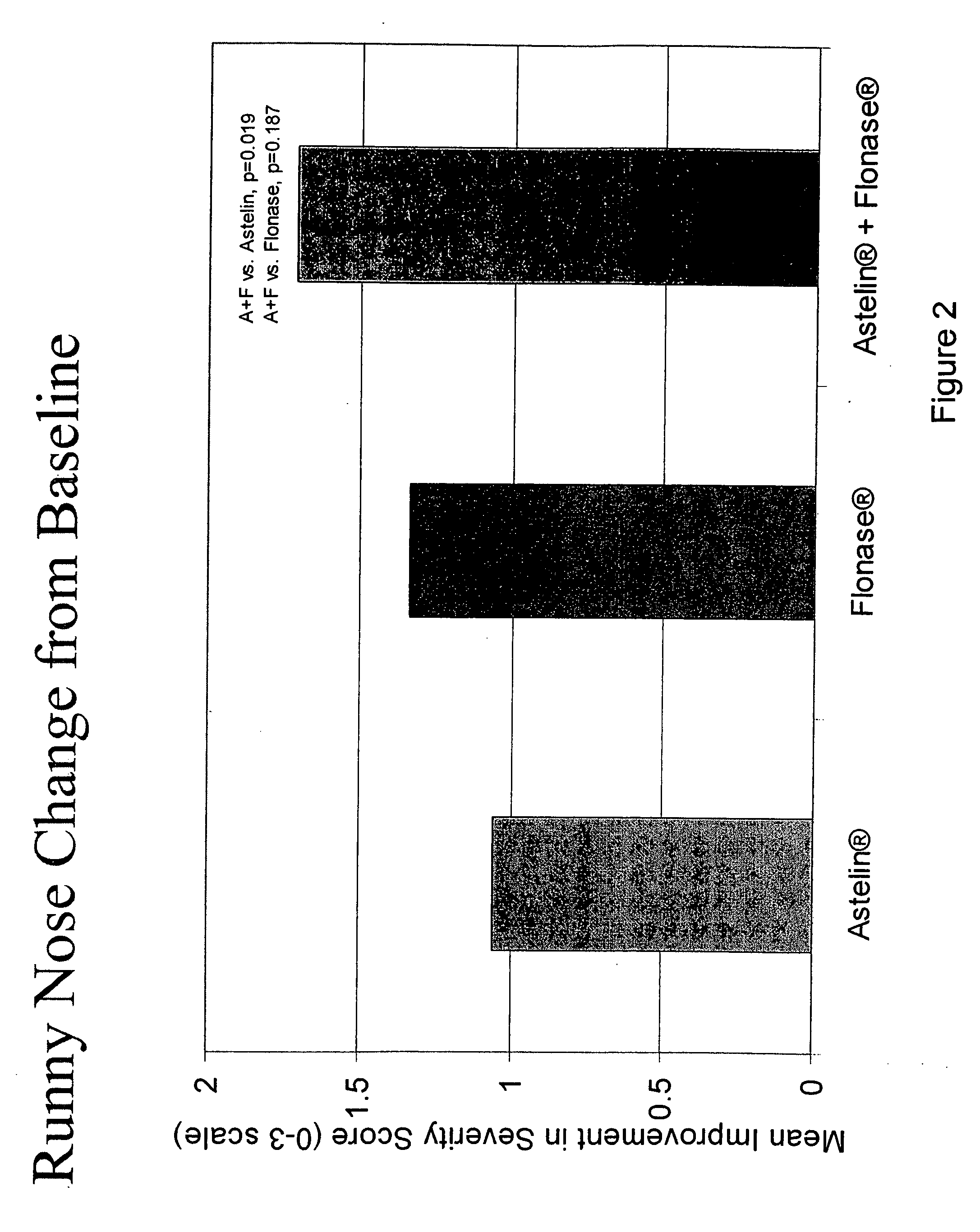
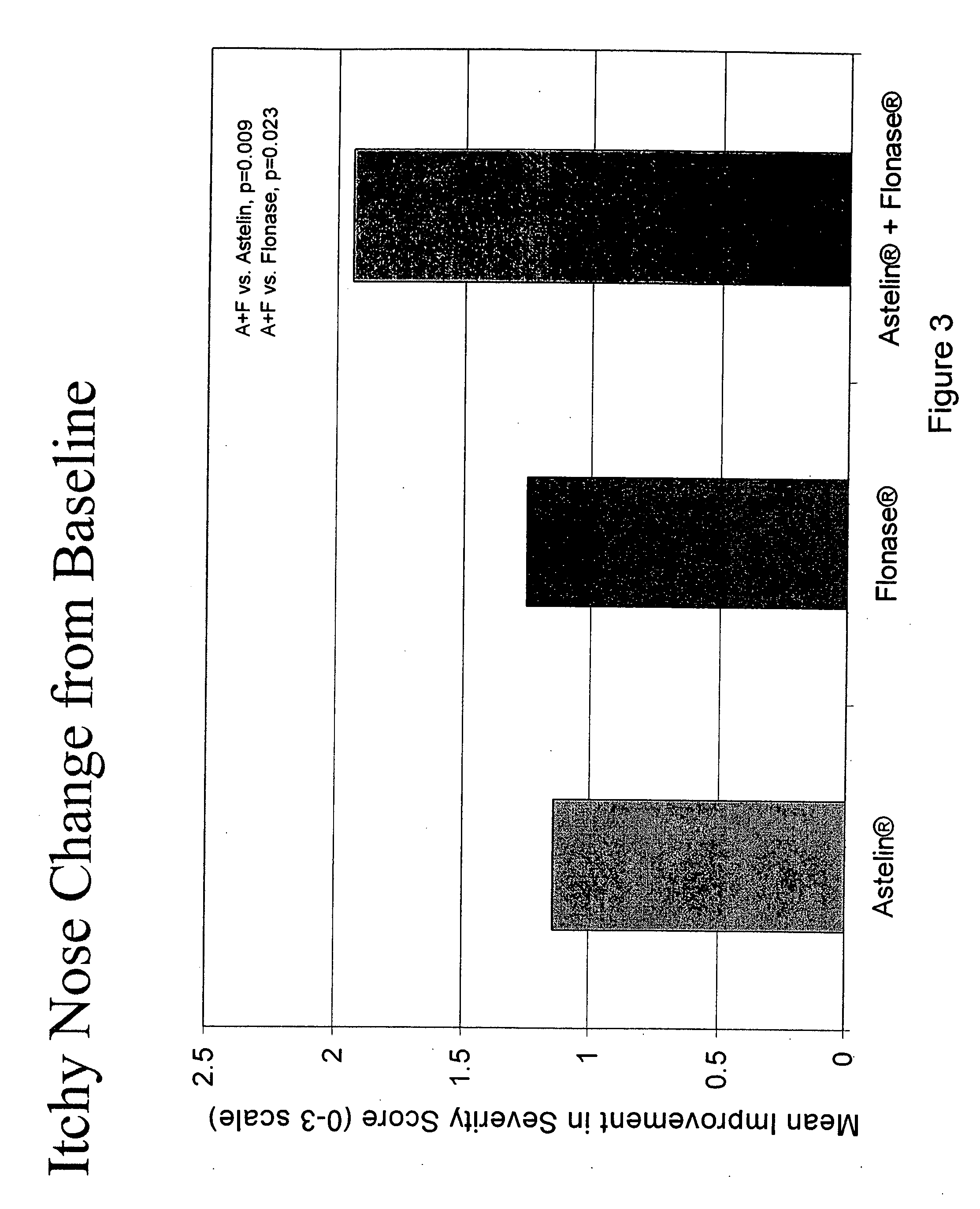
Compositions comprising azelastine and methods of use thereof
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Azelastine Hydrochloride Nasal Solution
[1224] In one exemplary composition of the invention, a nasal spray formulation containing azelastine hydrochloride was prepared using hypromellose as a thickener, sorbitol as an isotonicity agent and sweetener, and sucralose as both a sweetener and a taste-masking agent.
Ingredient%Azelastine Hydrochloride0.150Hypromellose 2900, USP 40000.100Edetate Disodium, USP0.050Sorbitol 70%, USP6.400Sodium Citrate, USP, Dihydrate0.068Sucralose, NF0.150Benzalkonium Chloride 50% Solution, NF0.025Water Purified or DeionizedQ.S. to 100%
[1225] Following preparation, the composition was filtered, and was packaged into high density polyethylene bottles fitted with a screw cap and comprising a VP3 metered-dose spray pump designed for intranasal application in a volume of about 0.14 ml (Valois). For use, one or two sprays were administered to each nostril two times per day, or as prescribed.
example 2
Azelastine Hydrochloride Nasal Solution
[1226] In another exemplary composition provided by the present invention, a nasal spray formulation containing azelastine hydrochloride was prepared using hypromellose as a thickener and sucralose and menthol as taste-masking agents:
Ingredient%Azelastine Hydrochloride0.100Hypromellose 2900, USP 40000.300Edetate Disodium, USP0.050Sodium Citrate, USP, Dihydrate0.068Sucralose, NF0.050Propylene Glycol, USP1.895Menthol, USP0.050Benzalkonium Chloride 50%, NF0.025Water Purified or DeionizedQ.S. to 100%
[1227] Following preparation, the composition was filtered, and was packaged into high density polyethylene bottles fitted with a screw cap and comprising a VP3 metered-dose spray pump designed for intranasal application in a volume of about 0.14 ml (Valois). For use, one or two sprays were administered to each nostril two times per day, or as prescribed.
example 3
Azelastine Hydrochloride Nasal Solution
[1228] In another exemplary composition of the invention, a nasal spray formulation containing azelastine hydrochloride was prepared using hypromellose as a thickener, sodium chloride as an isotonicity agent, and sucralose as both a sweetener and a taste-masking agent.
Ingredient%Azelastine Hydrochloride0.100Hypromellose 2900, USP 40000.100Edetate Disodium, USP0.050Citric Acid Anhydrous, USP0.044Dibasic Sodium Phosphate Heptahydrate, USP0.486Sodium Chloride, USP0.687Sucralose, NF0.150Benzalkonium Chloride 50% Solution, NF0.025Water Purified or DeionizedQ.S. to 100%
[1229] Following preparation, the composition was filtered, and was packaged into high density polyethylene bottles fitted with a screw cap and comprising a VP3 metered-dose spray pump designed for intranasal application in a volume of about 0.14 ml (Valois). For use, one or two sprays were administered to each nostril about two times per day, or as prescribed.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
| Percent by mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com