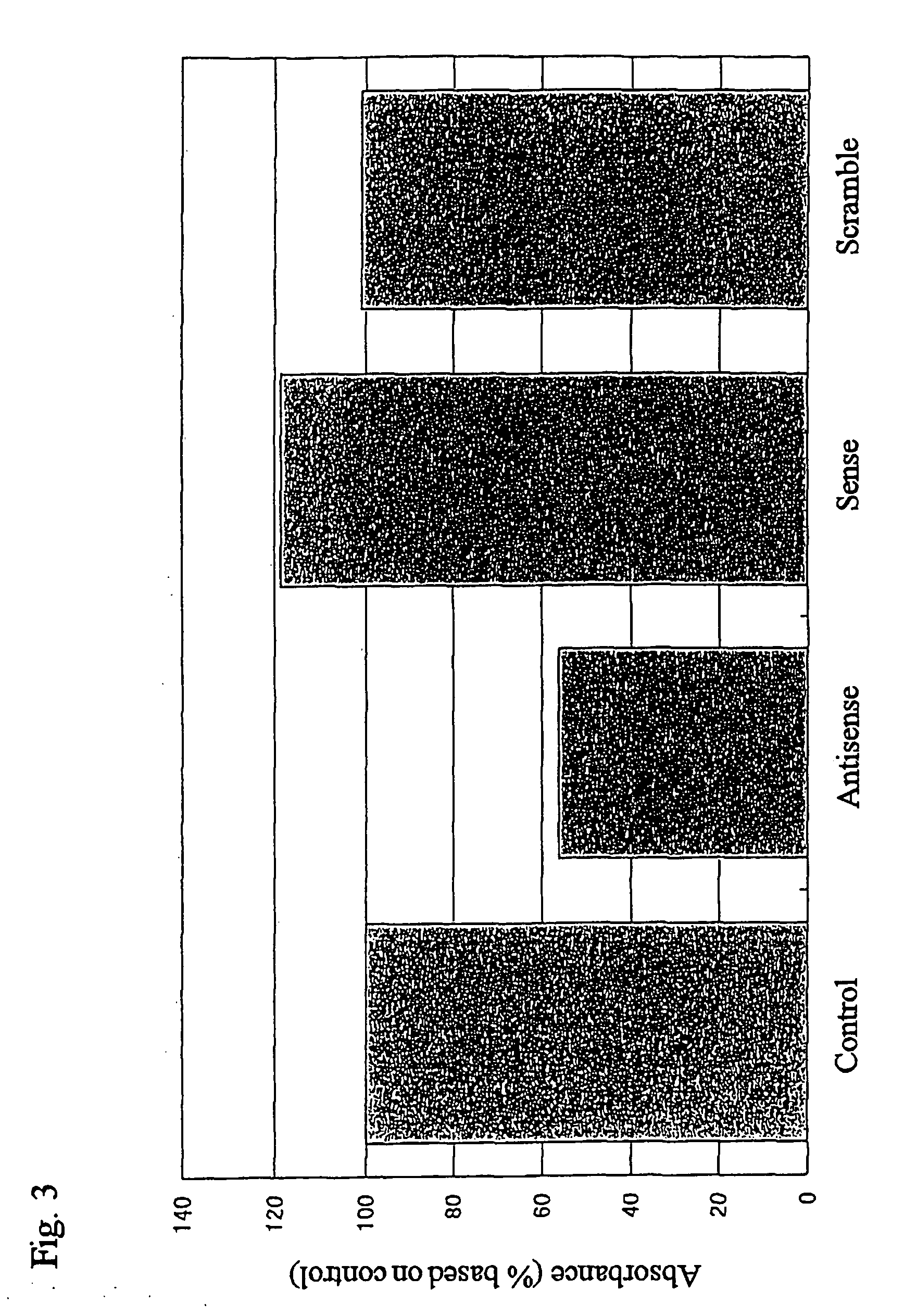
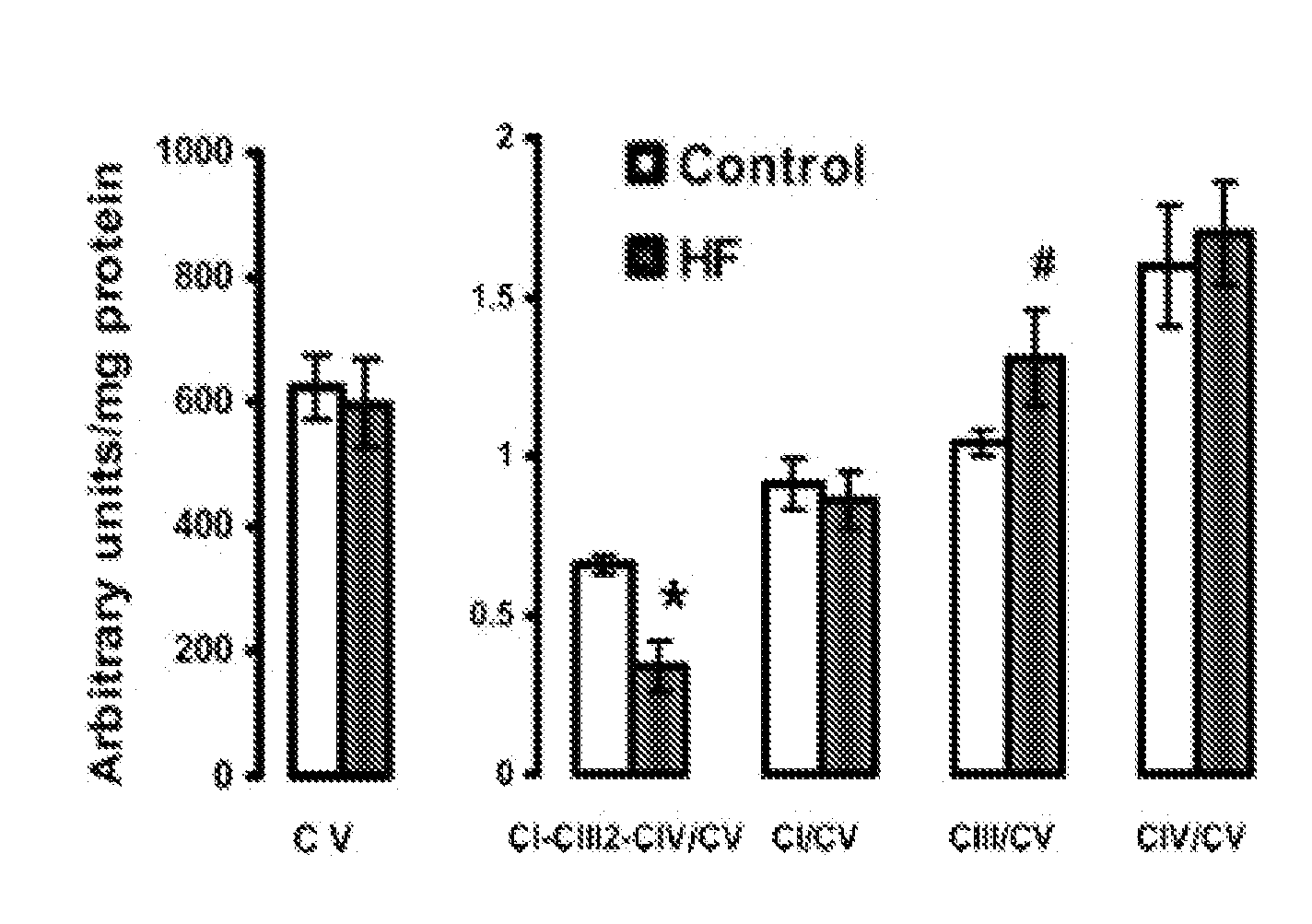
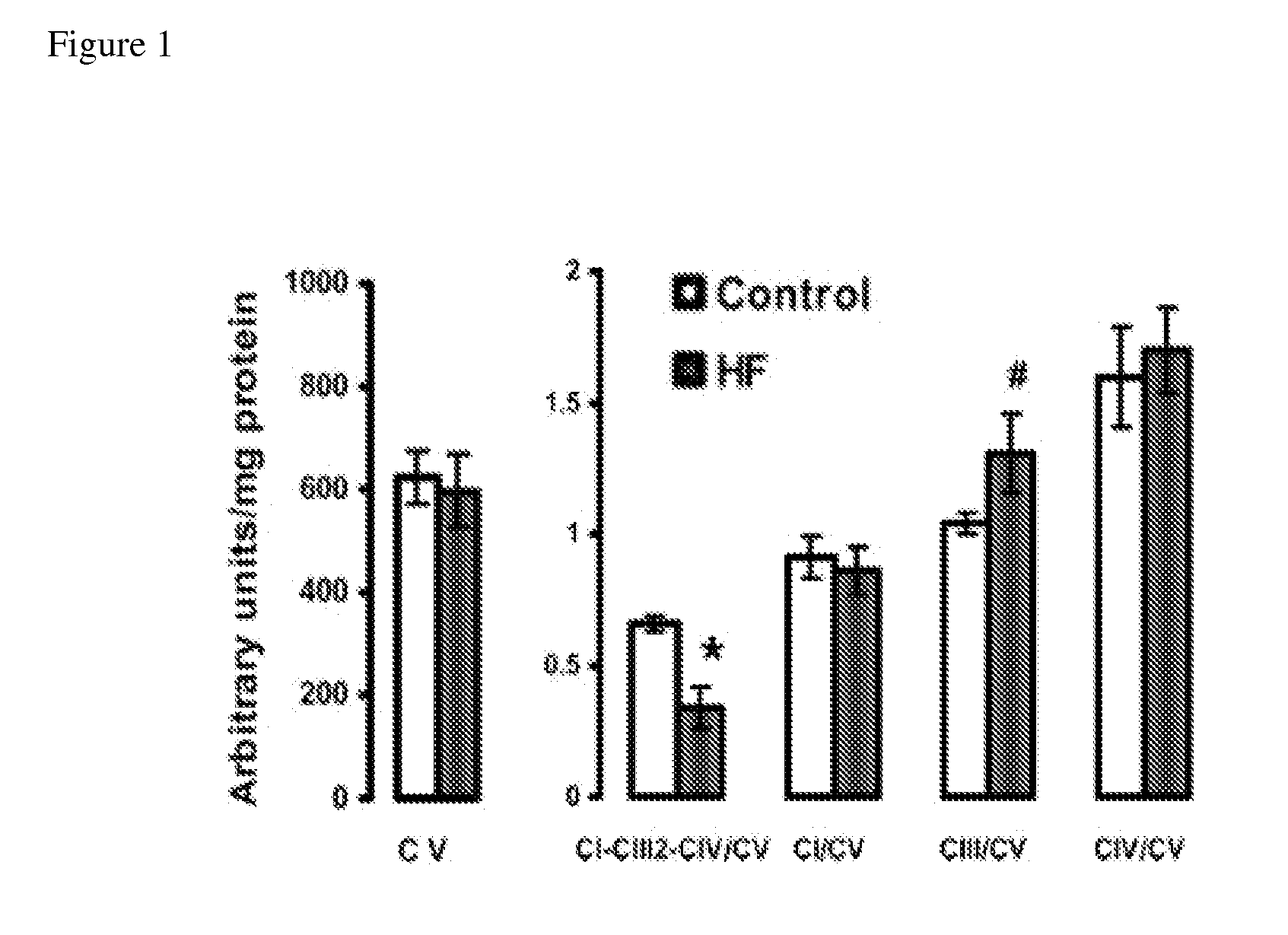
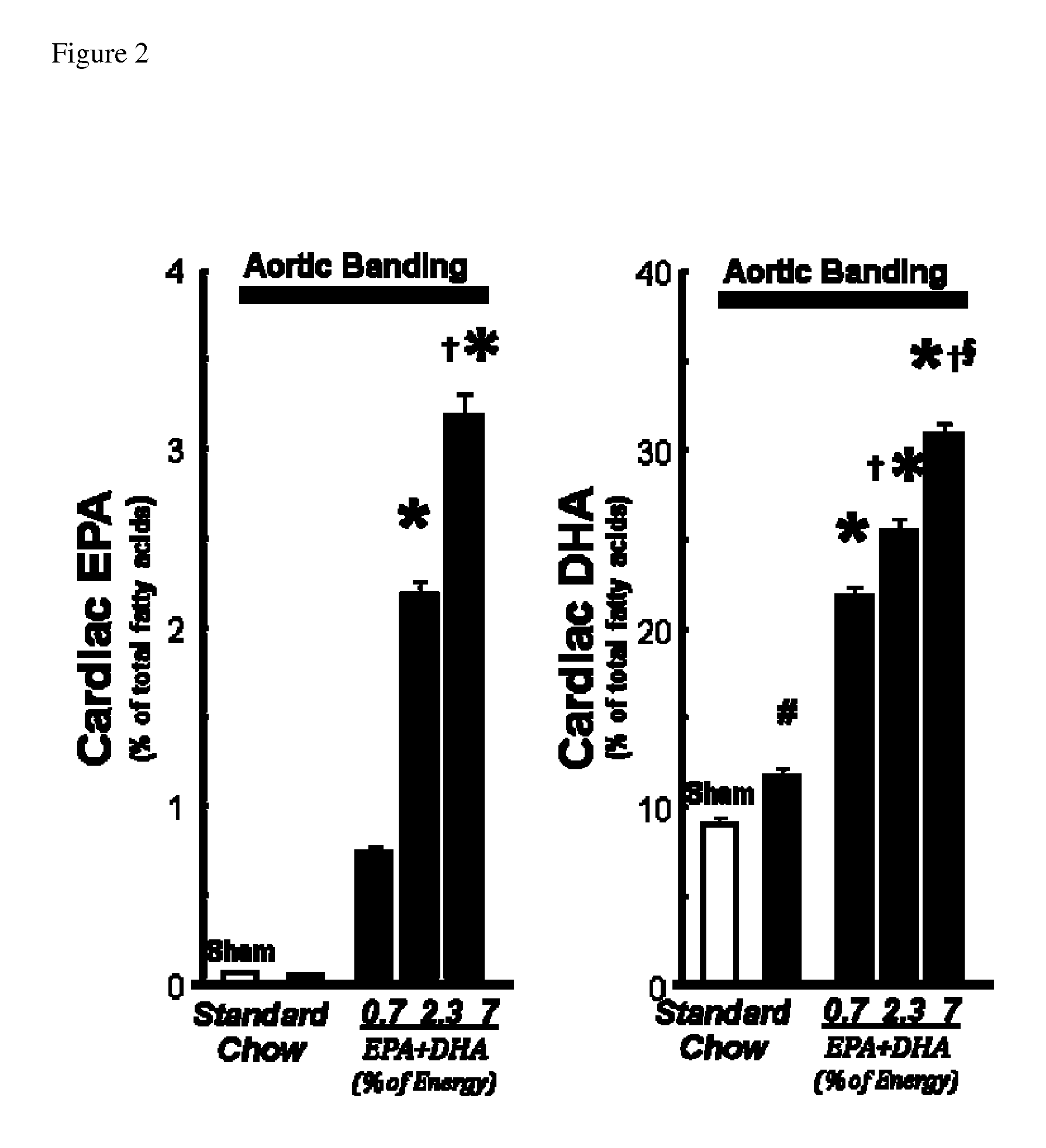
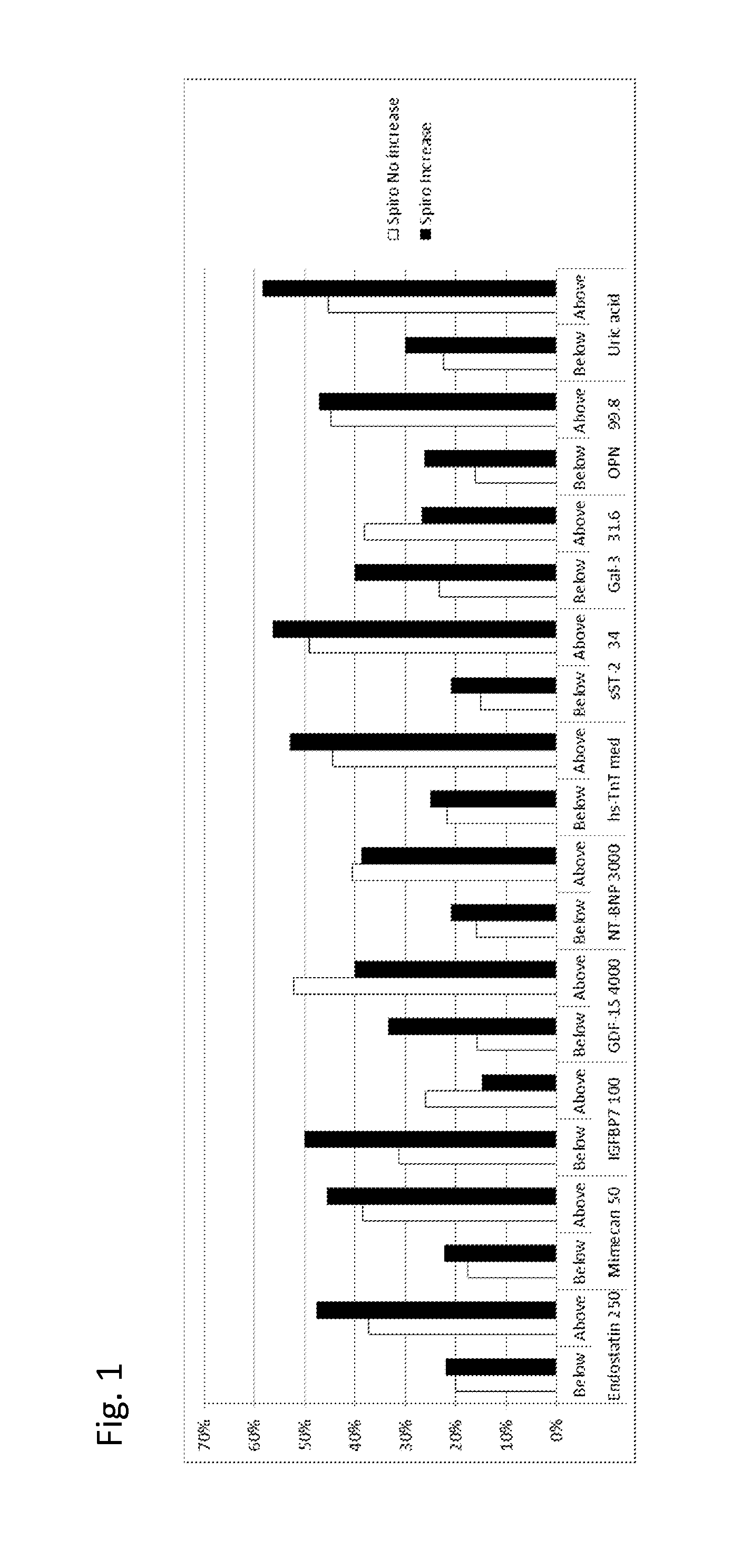
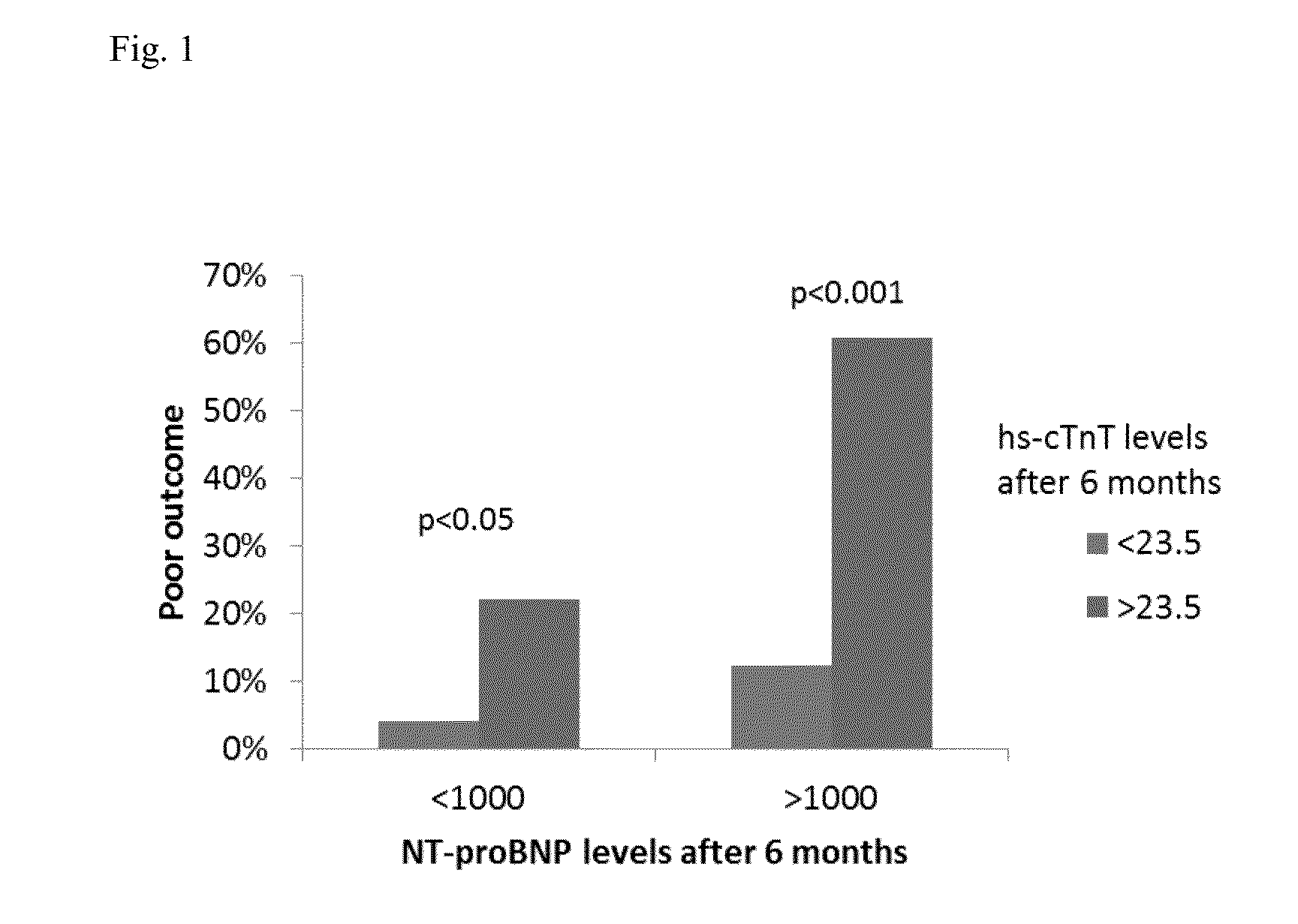Patents
Literature
37 results about "Cardiac failure therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
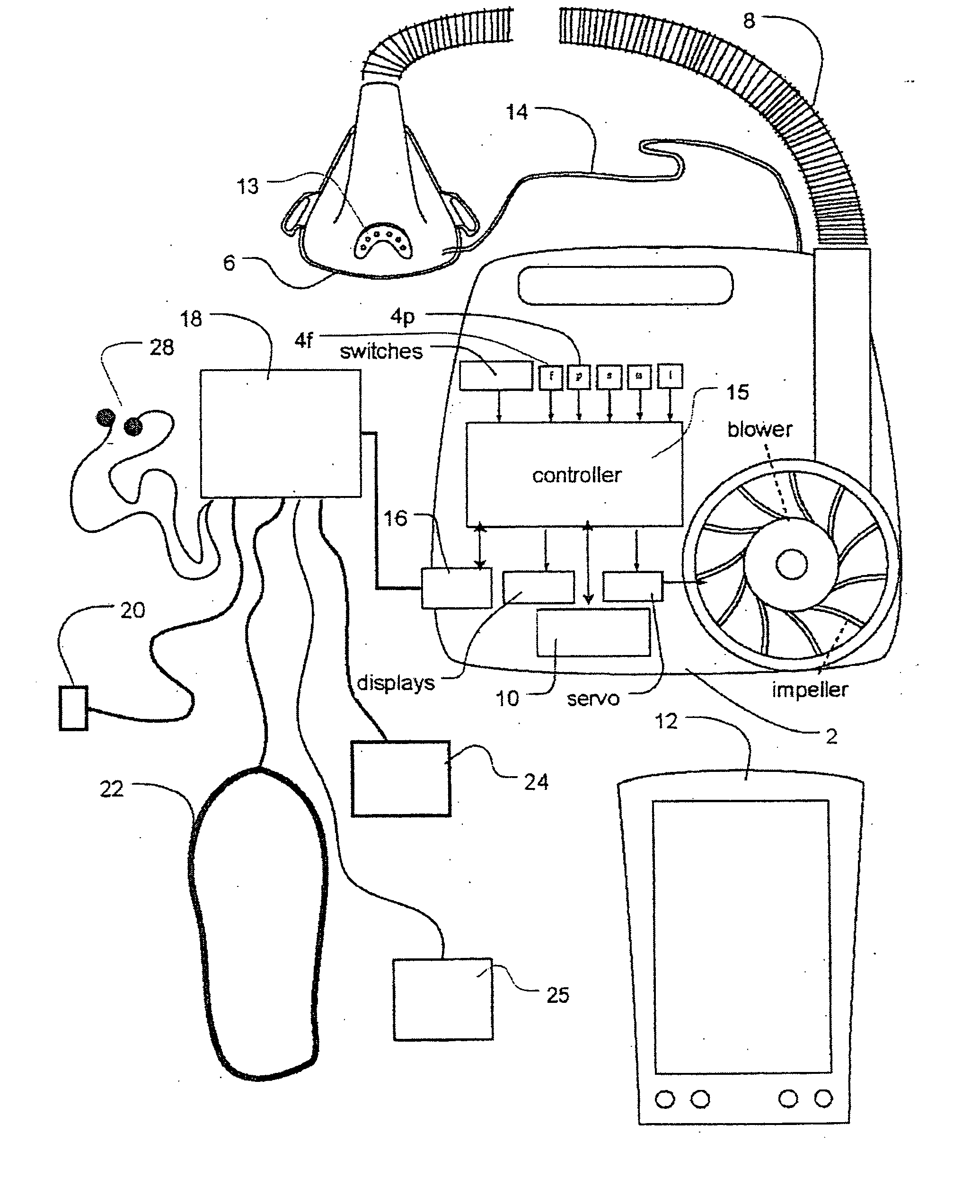
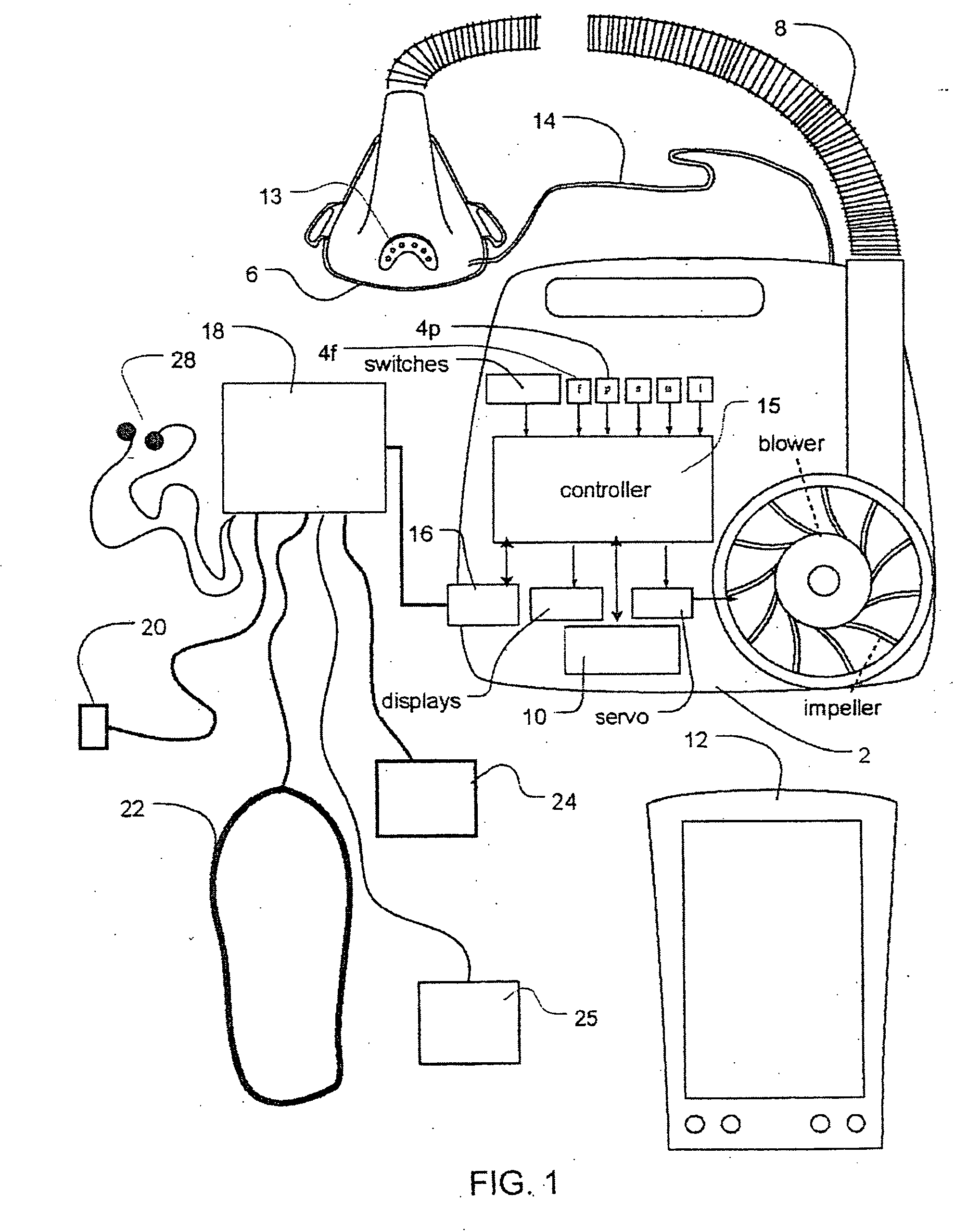
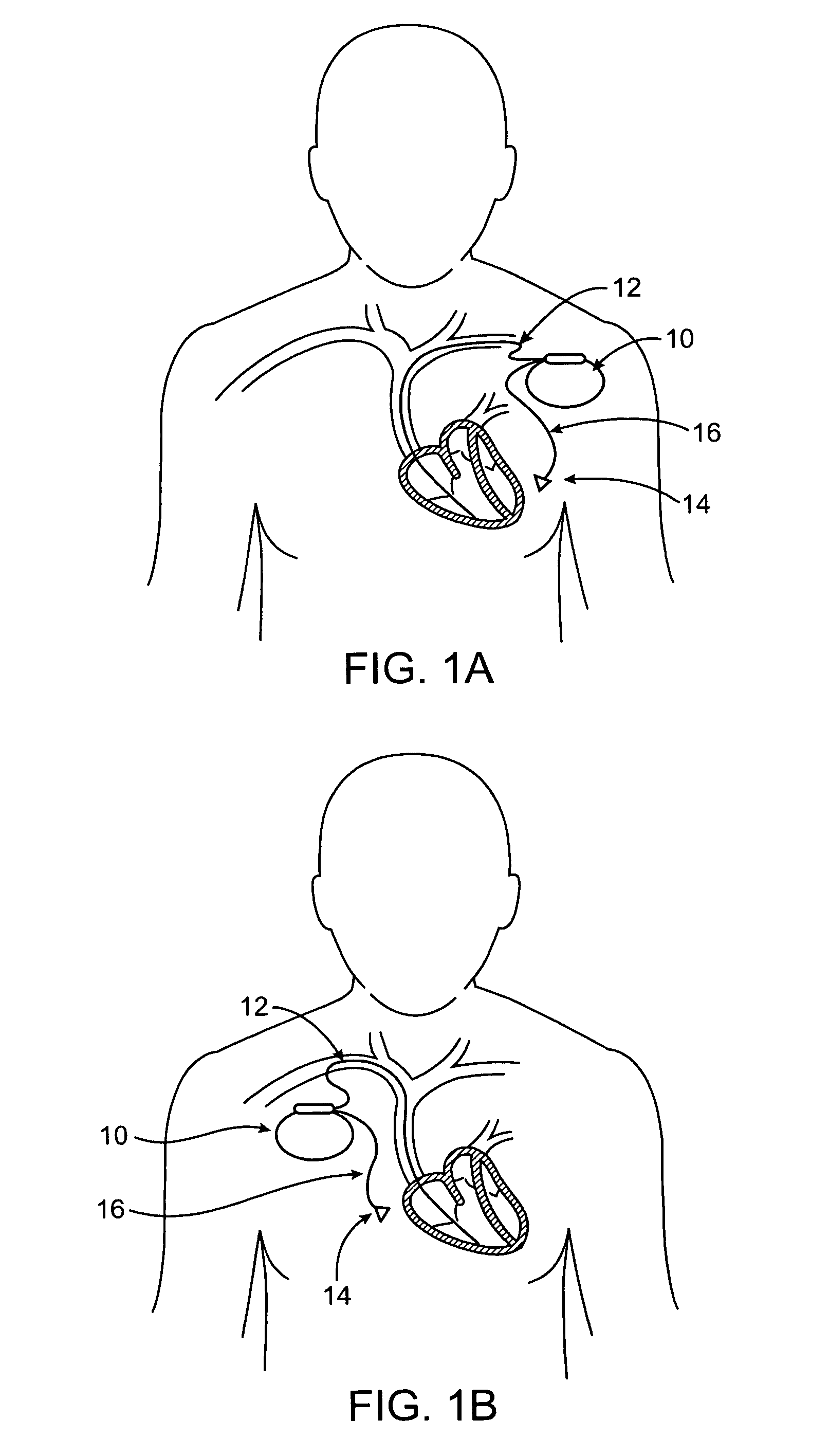
Methods and apparatus for heart failure treatment
InactiveUS20060084877A1Treatment level is not increasedIncrease pressureElectrocardiographyRespiratory masksCardiac failure therapyDisease cause
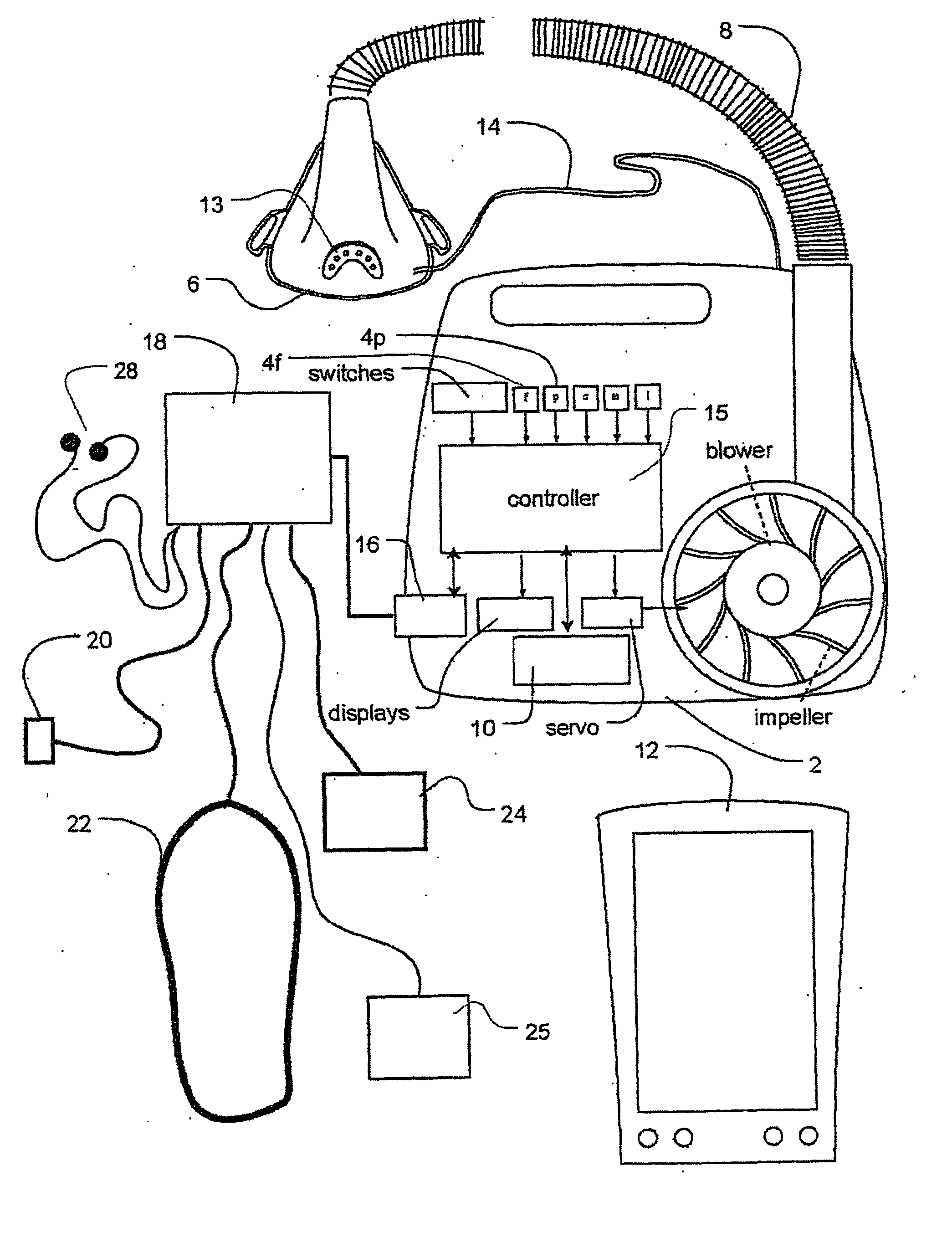
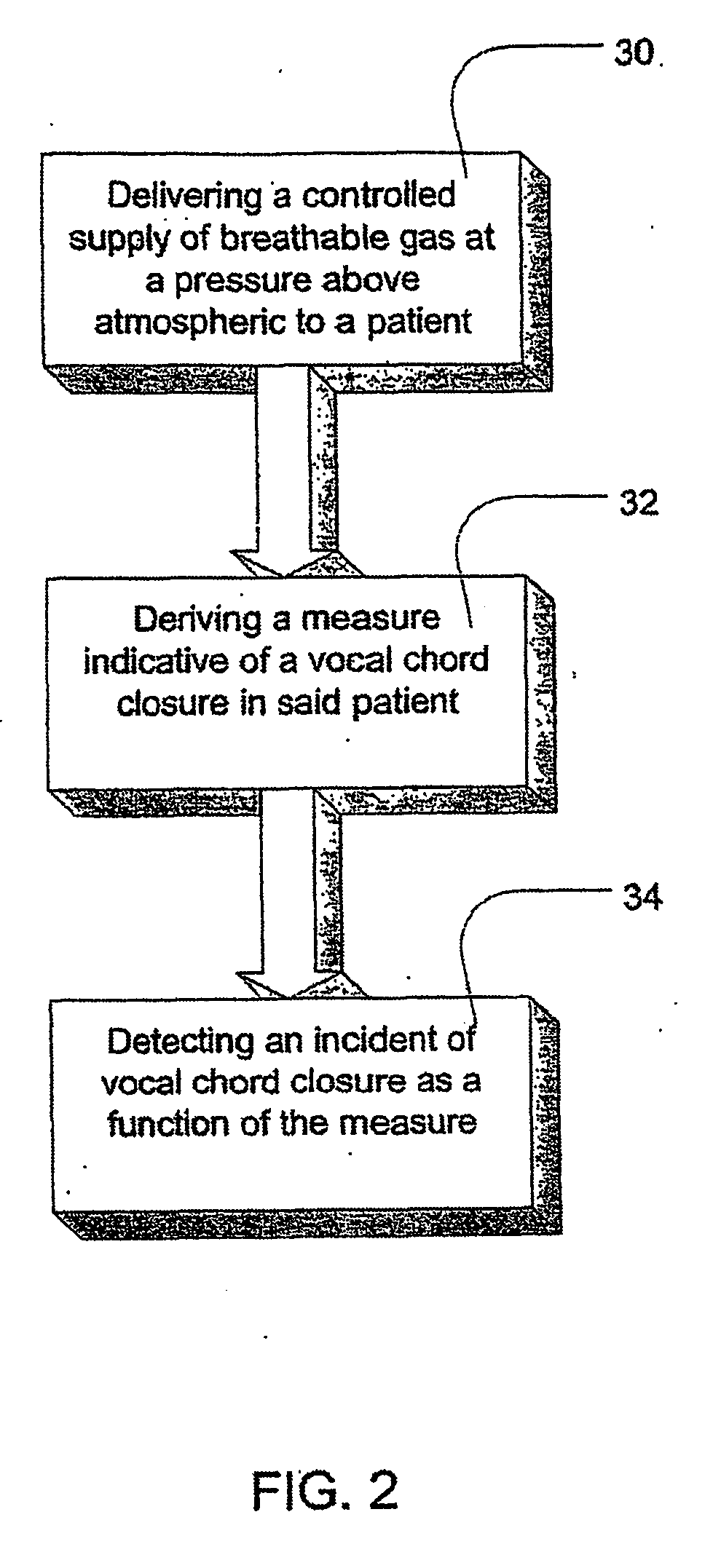
Methods and apparatus for assessing the condition of and treating patients for heart failure by the delivery of continuous positive airway pressure are disclosed. Airflow of the patient is measured to determine occurrences of central apneas. A heart failure index is calculated from a count of the number of central apneas that have occurred. The present heart failure index can be compared with a previously calculated heart failure index to determine how the patient's heart failure disease has changed and how it should be treated.
Owner:RESMED LTD
Vibrational therapy device used for resynchronization pacing in a treatment for heart failure
Systems for pacing the heart include a vibrational transducer which directs energy at the heart, usually at at least a ventricle, to pace the heart and to promote synchronized contraction of the ventricles. Optionally, additional vibrational and / or electrical stimulation may be provided. The vibrational transducers are usually implantable at a location proximate the heart.
Owner:EBR SYST
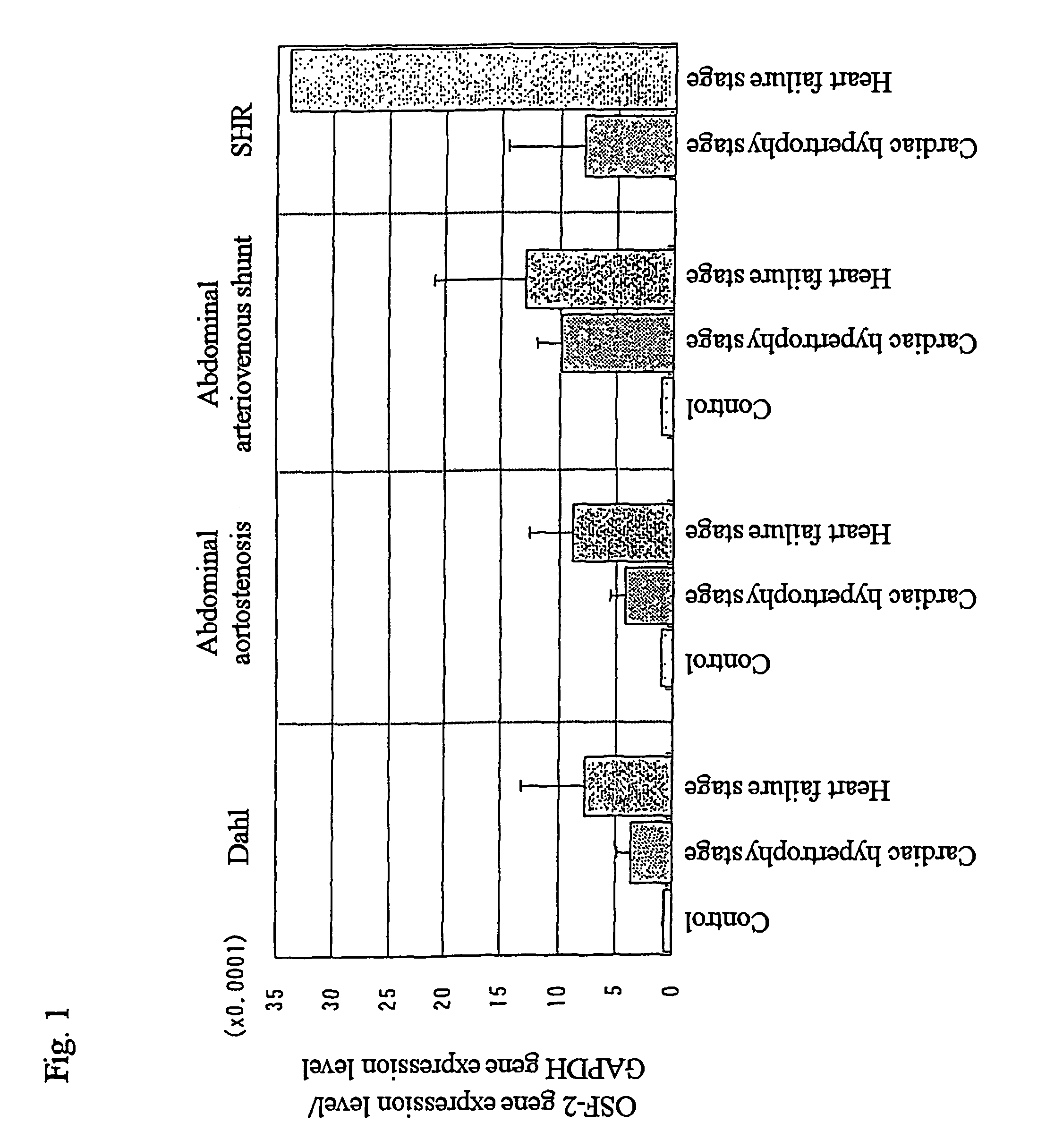
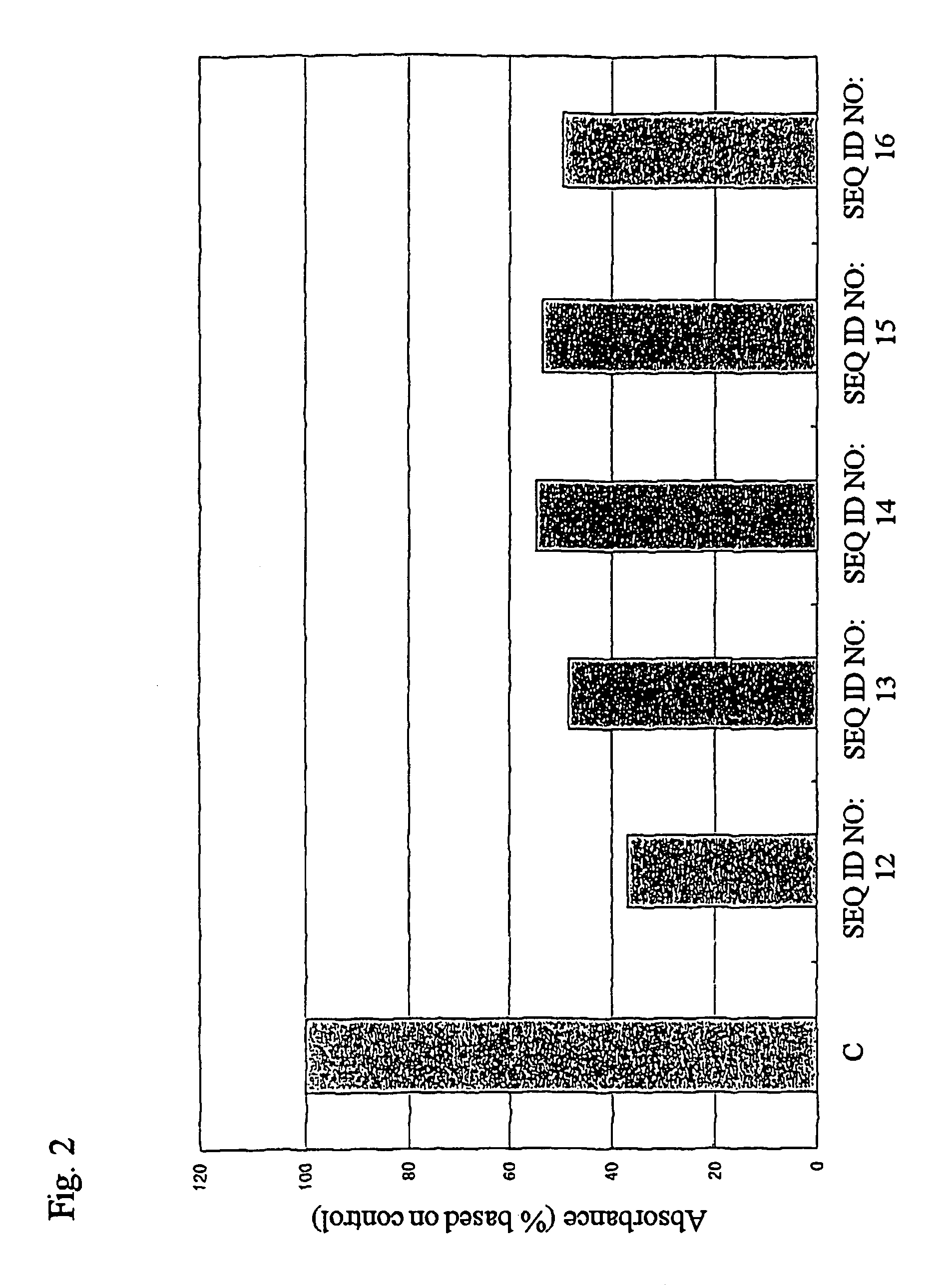
Remedies for heart failure
InactiveUS7816511B2Preventing and treating heart failureInhibit deteriorationBiocideSugar derivativesCardiac failure therapyPharmaceutical drug
The present invention provides methods for screening drugs inhibiting the expression of OSF-2 gene or the production or function of the protein encoded thereby and therapeutic agents for heart failure having such effects. Useful methods for diagnosing heart failure can be provided by monitoring the expression or variation of said gene or the production of the protein encoded thereby. The present invention also provides transgenic animals with forced expression of OSF-2 gene and methods for studying changes in gene expression or protein production or the functions of various genes or proteins with the progress of the pathology of heart failure using them and novel therapeutic agents for heart failure.
Owner:ASUBIO PHARMA
Heart failure treatment device and method
Owner:PARACOR MEDICAL INC
Heart failure treatment device and method
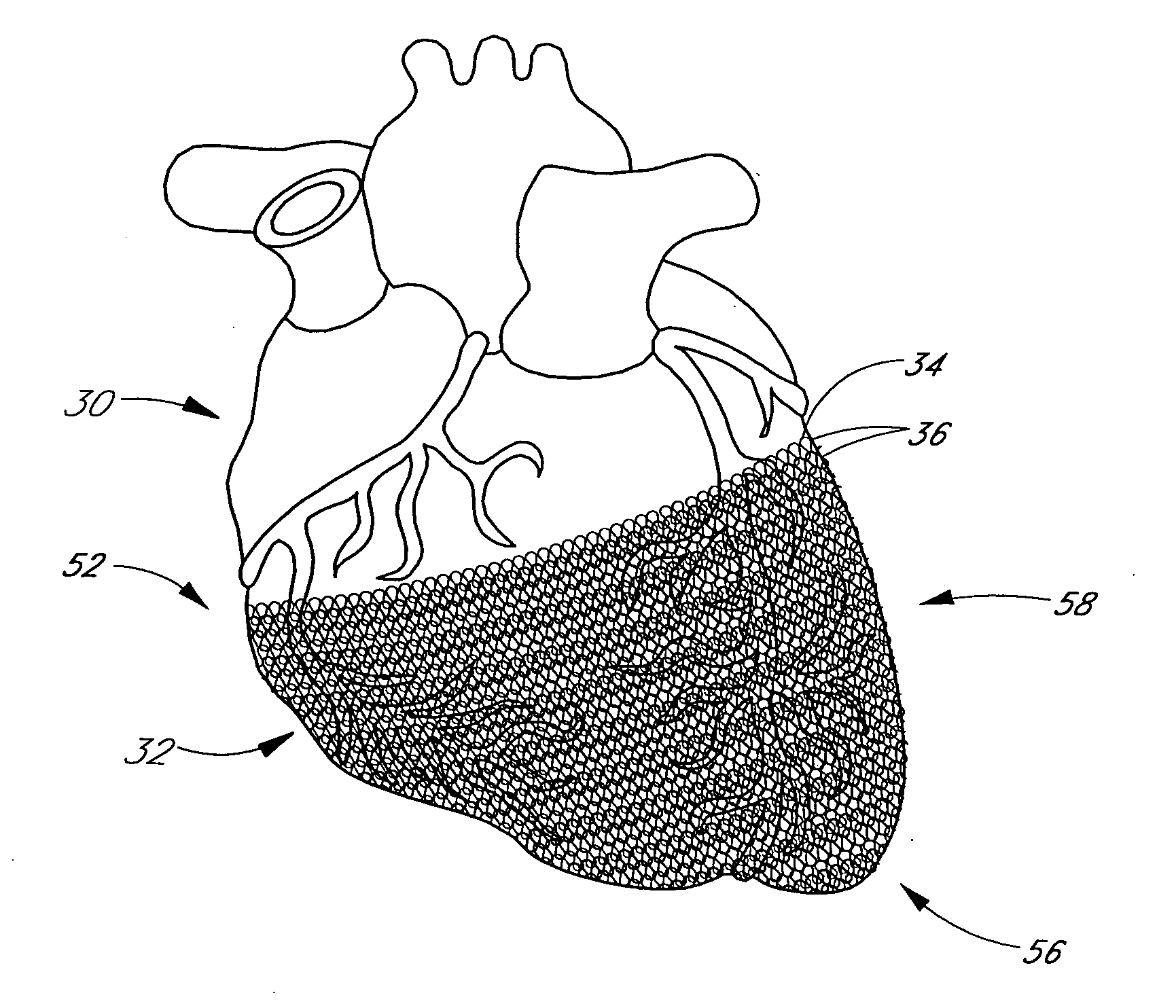
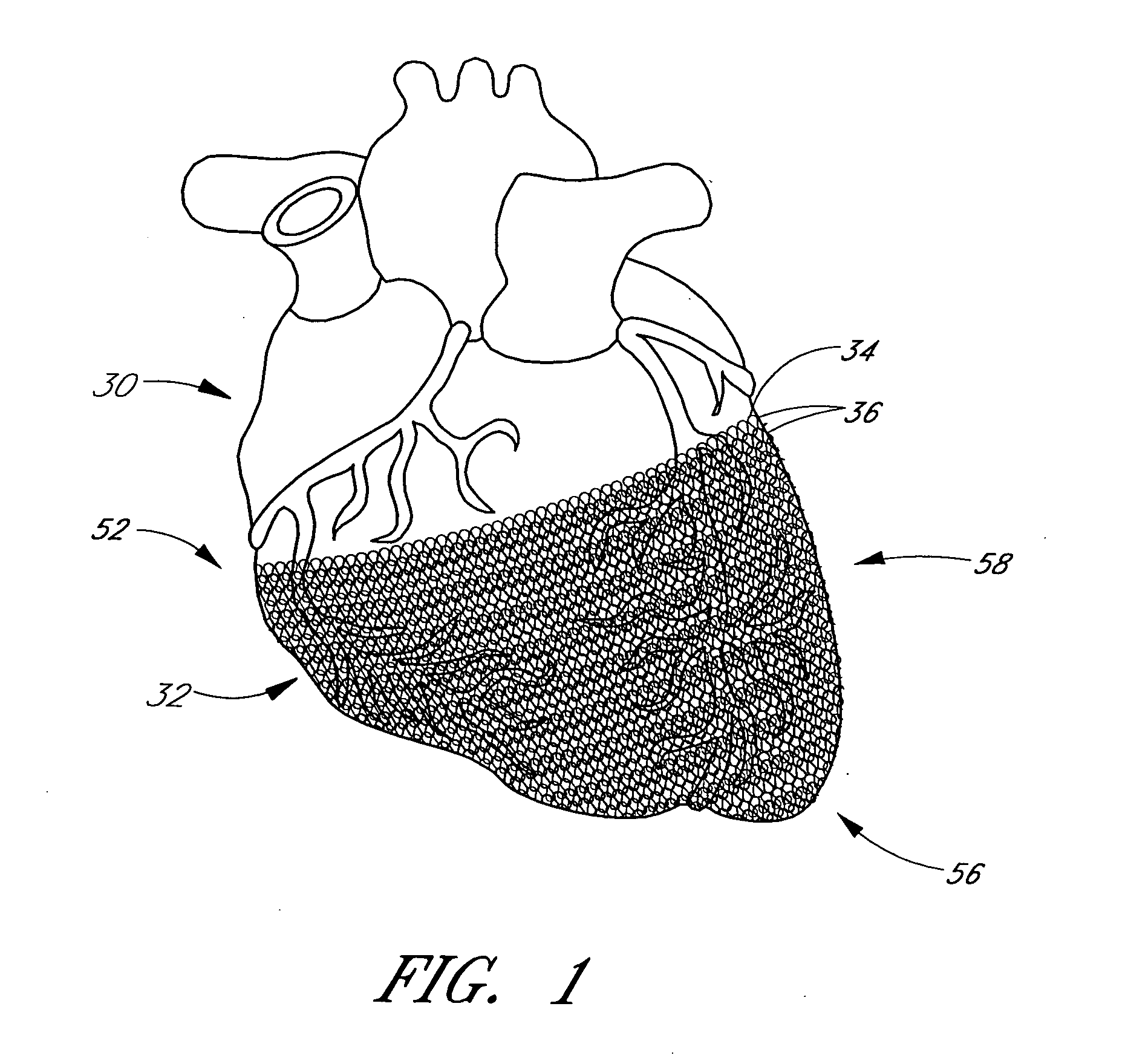
A method and apparatus for treating heart failure is configured to be placed about at least a portion of a patient's heart to apply a mild compressive force on the heart over a range of elastic deformation of the apparatus. The apparatus can be shifted to second range of deformation. In some embodiments, the apparatus is shifted to the second range of deformation by application of a stimulus or alteration of environmental conditions beyond a threshold level.
Owner:PARACOR MEDICAL
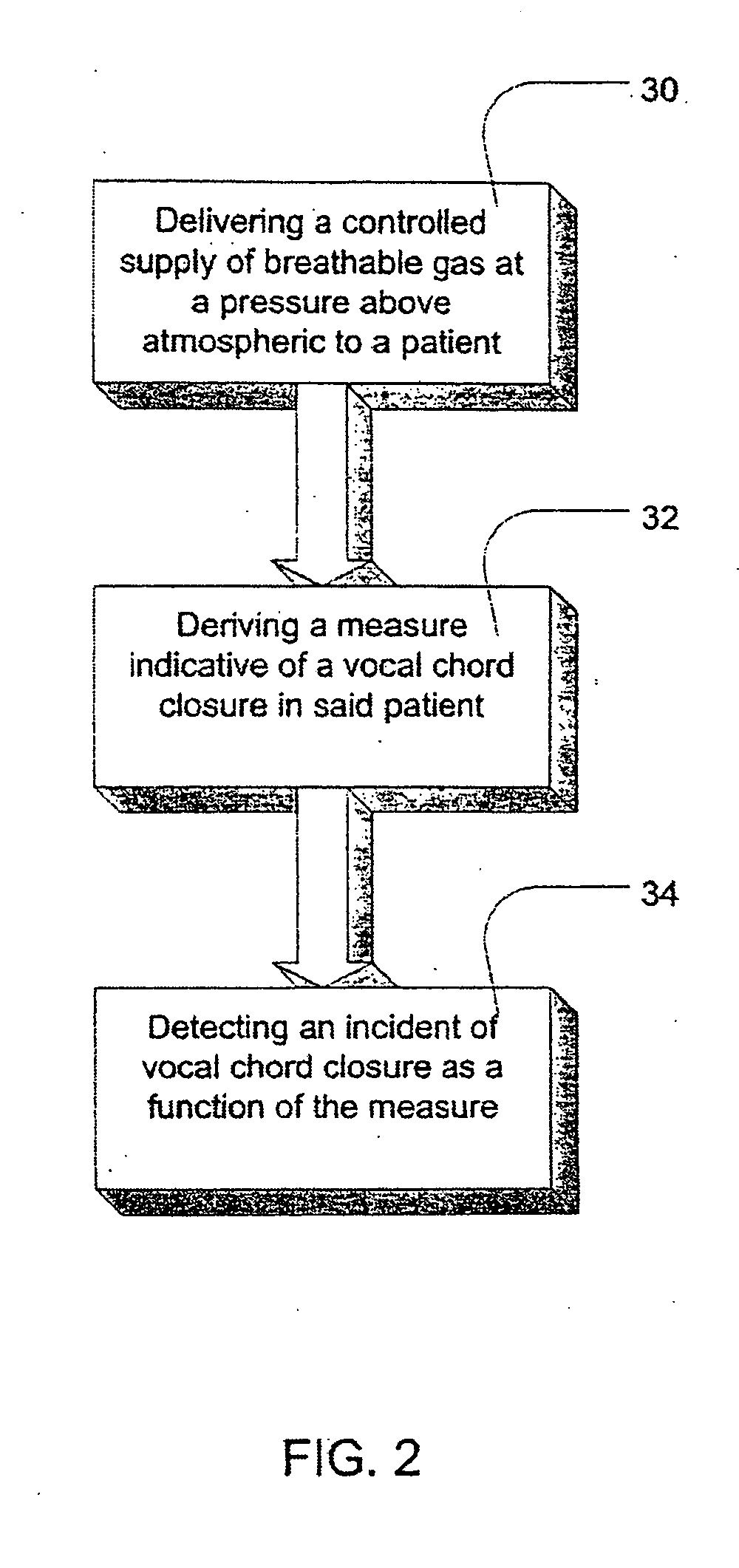
Methods and apparatus for heart failure treatment
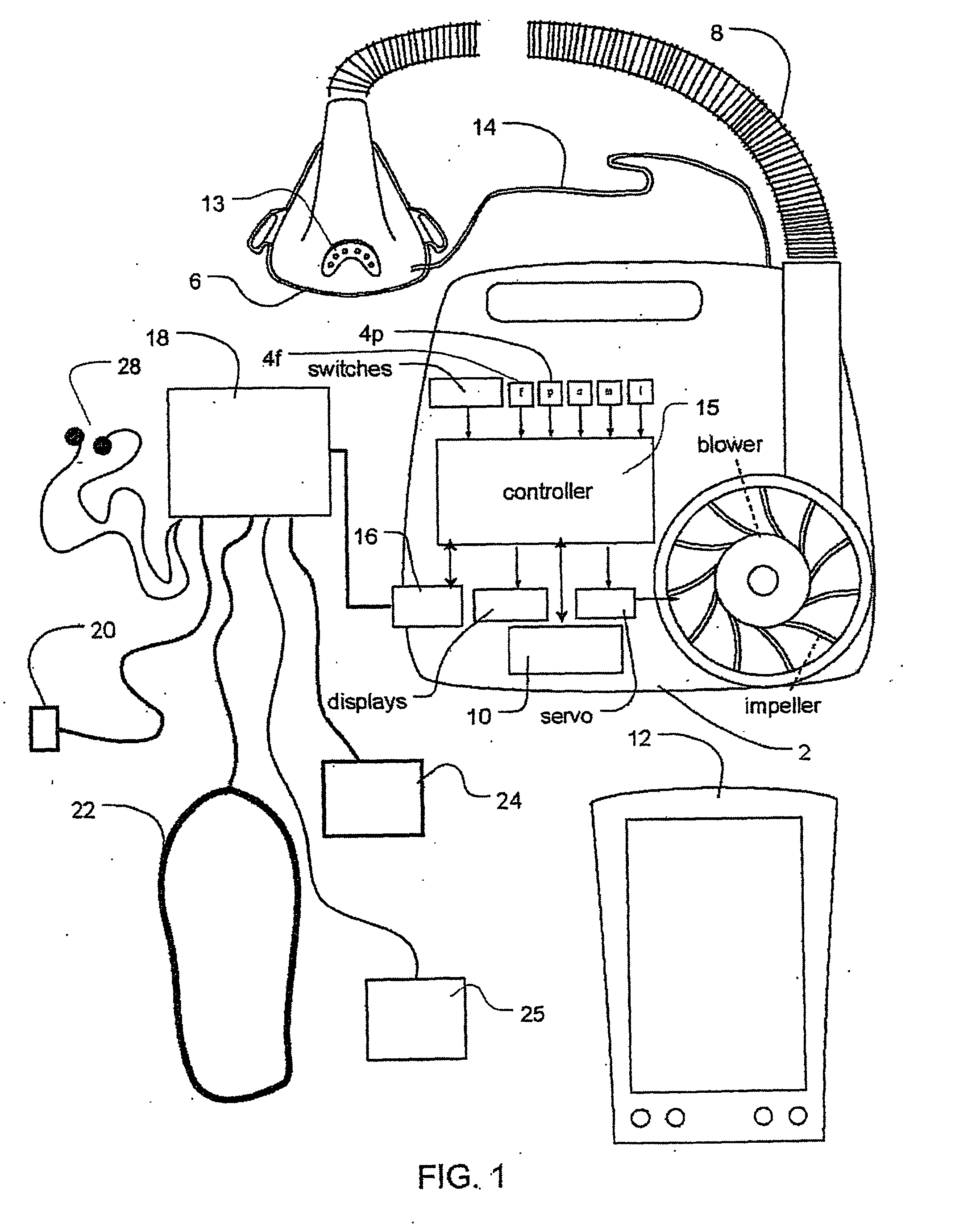
Methods and apparatus for assessing the condition of and treating patients for heart failure by the delivery of continuous positive airway pressure are disclosed. Treatment of obstruction due to reflex vocal cord closure often experienced by heart failure patients is distinguished from treatment of upper airway obstruction typically associated with Obstructive Sleep Disorder. Treatment may also be implemented by delivering synchronized cardiac pressure oscillations superimposed on a respiratory pressure level to provide assistance for the heart. Heart treatment pressure dose indicator may be calculated for prescribing and monitoring the delivery of treatment. The apparatus may also generate data to track heart failure condition that may be indicative of the degree of severity of heart failure based upon breathing patterns to assist in the diagnosis and management of heart failure patients.
Owner:UJHAZY ANTHONY JOHN +4
Neuregulin based methods for treating heart failure
ActiveUS20130079281A1Improves and protects deteriorationImprove survivalPeptide/protein ingredientsAntipyreticNeuregulinHeart failure cell
The present invention features methods of treating patients with chronic heart failure by administering a neuregulin polypeptide within a dosage range which is both effective and safe.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
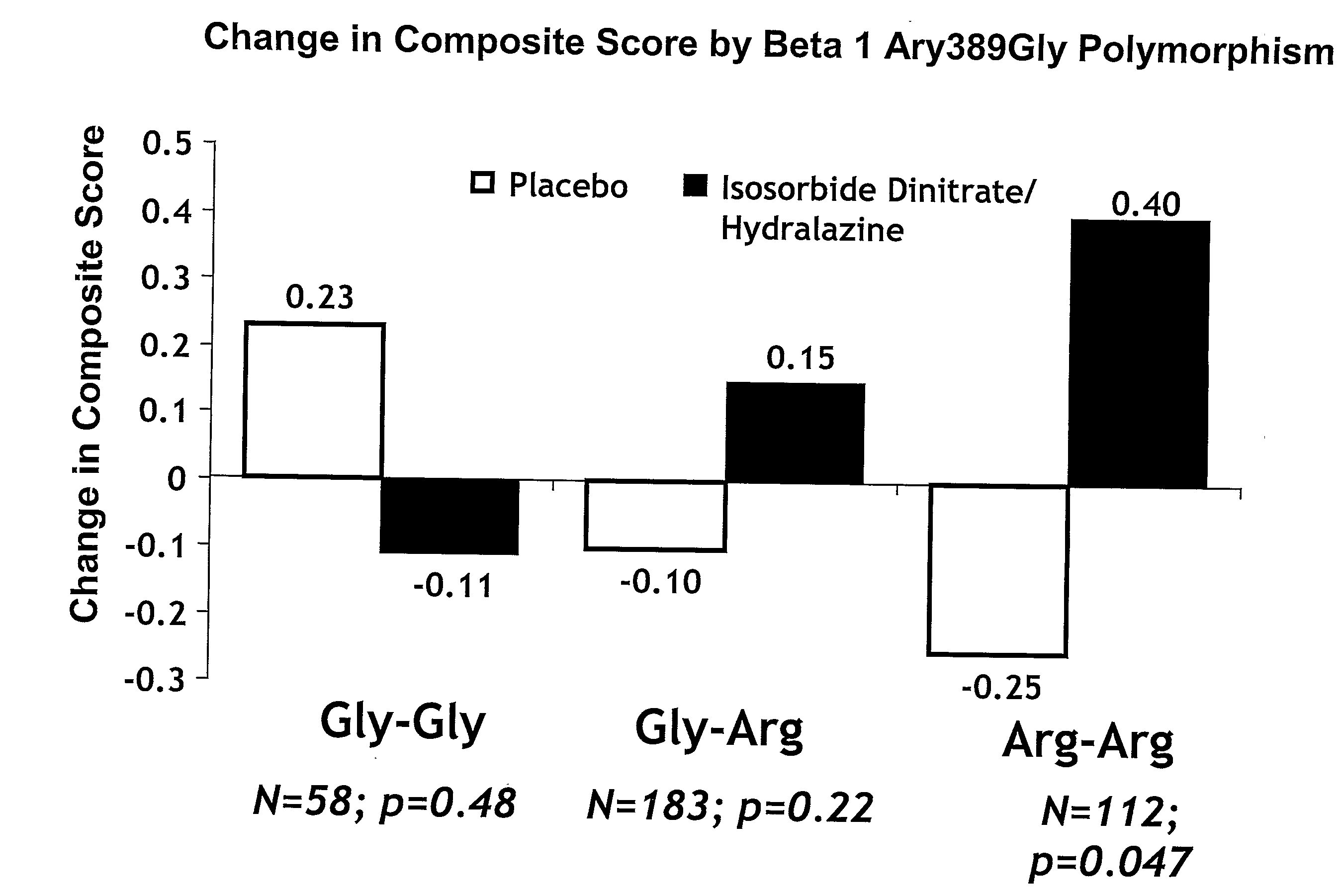
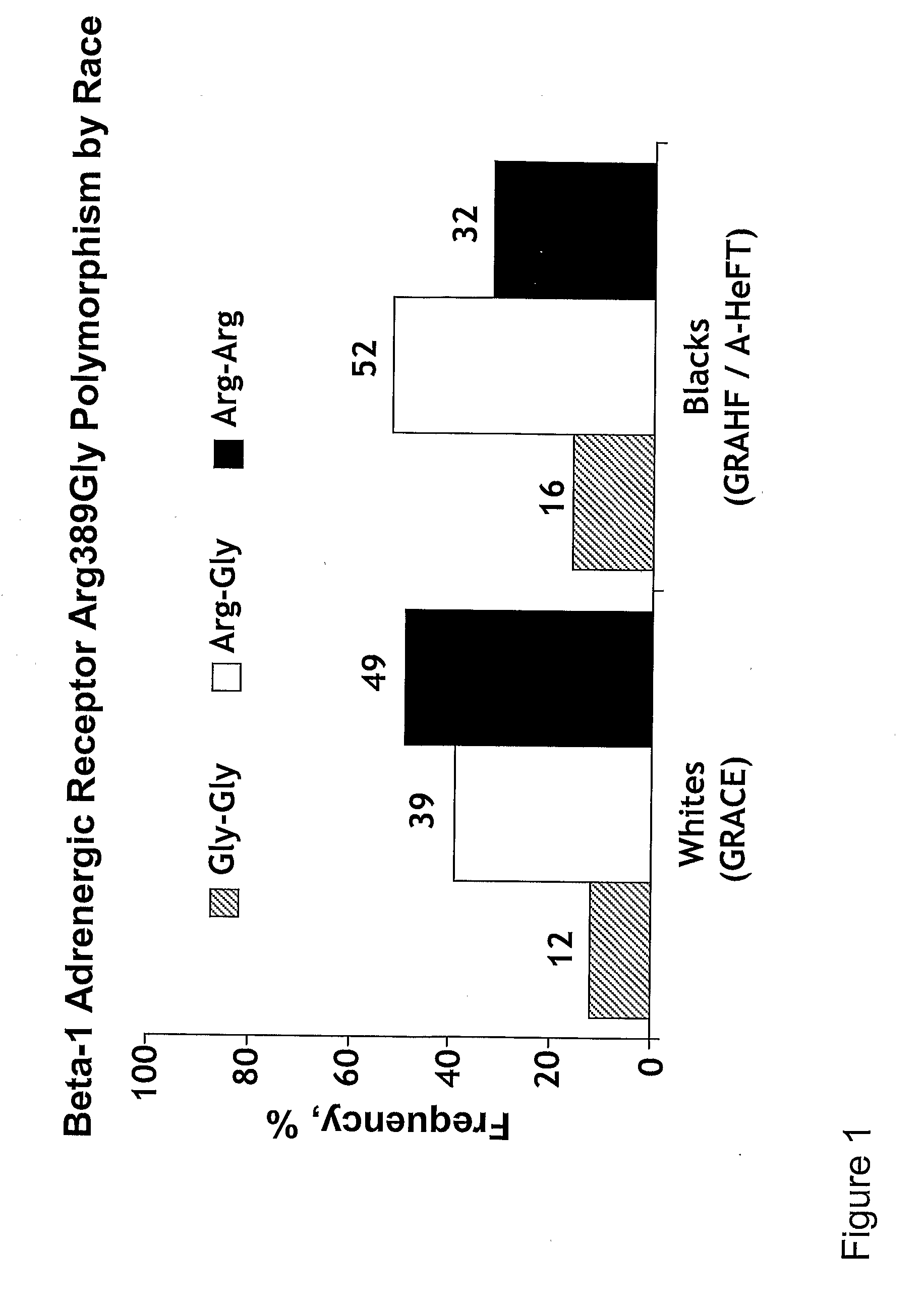
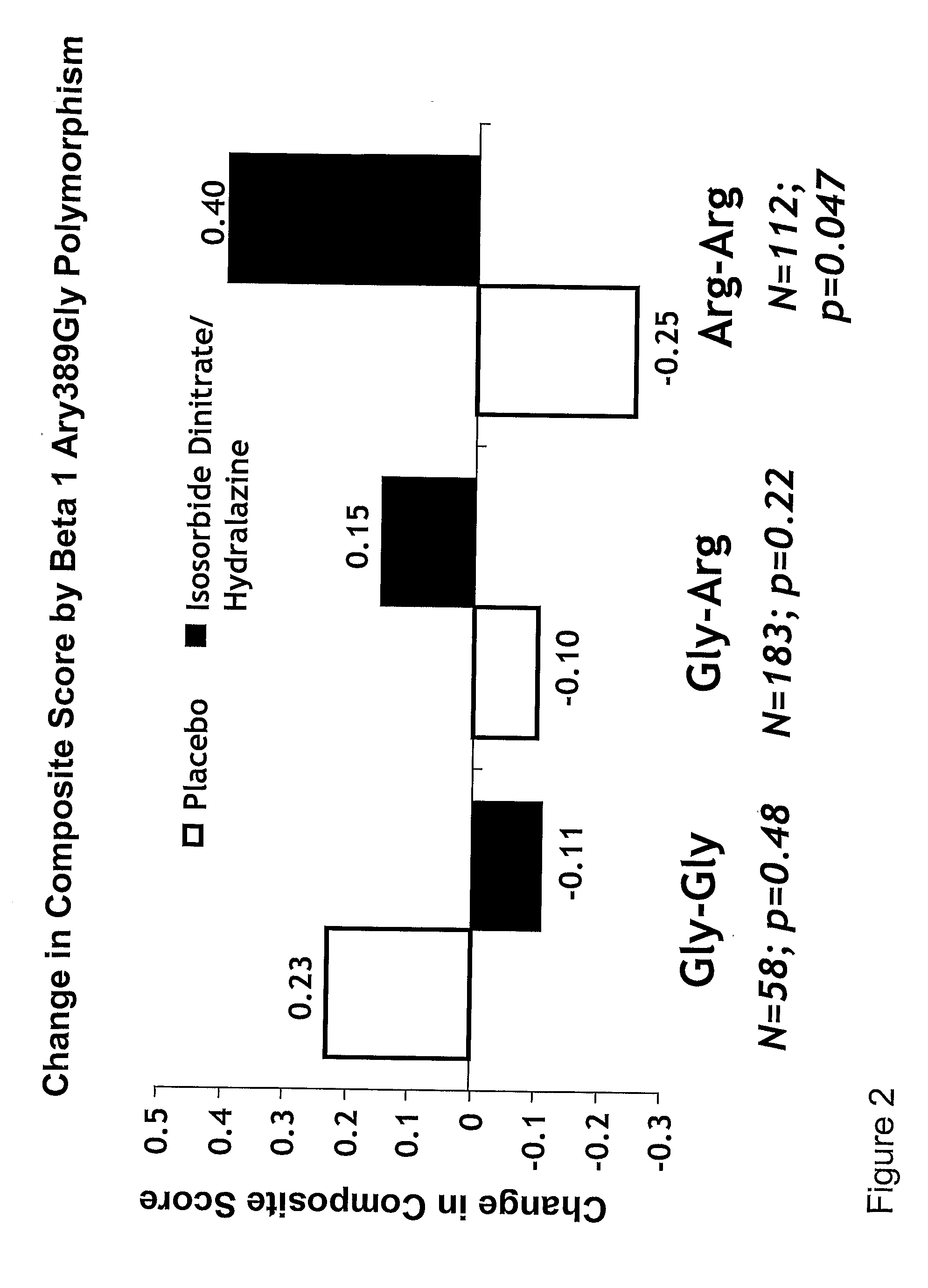
Genetic risk assessment in heart failure: impact of genetic variation of beta 1 adrenergic receptor gly389arg polymorphism
InactiveUS20090192128A1Reduce mortalityIncreased oxygen consumptionBiocideMicrobiological testing/measurementAntioxidantLeft ventricular size
The invention provides methods for (a) reducing mortality associated with heart failure; (b) improving oxygen consumption; (c) treating heart failure; (d) treating hypertension; (e) improving the quality of life in a heart failure patient; (f) inhibiting left ventricular remodeling; (g) reducing hospitalizations related to heart failure; (h) improving exercise tolerance; (j) increasing left ventricular ejection fraction; (k) decreasing levels of B-type natriuretic protein; (l) treating renovascular diseases; (m) treating end-stage renal diseases; (n) reducing cardiomegaly; (o) treating diseases resulting from oxidative stress; (p) treating endothelial dysfunctions; (q) treating diseases caused by endothelial dysfunctions; or (r) treating cardiovascular diseases; in a patient in need thereof, wherein the patient has a Arg389Arg polymorphism and / or a Gly389Gly polymorphism in the beta 1 adrenergic receptor gene, comprising administering to the patient (i) at least one antioxidant compound or a pharmaceutically acceptable salt thereof; (ii) at least one nitric oxide enhancing compound; and (iii) optionally the best current therapy for the treatment of cardiovascular diseases. In one embodiment the antioxidant is a hydralazine compound or a pharmaceutically acceptable salt thereof and the nitric oxide enhancing compound is isosorbide dinitrate and / or isosorbide mononitrate.
Owner:NITROMED +1
Compositions and methods for treating heart failure
InactiveUS20140364366A1Increase differentiationSimple structurePeptide/protein ingredientsNeuregulinsNeuregulinCardiac failure therapy
The present invention provides methods for treating chronic heart failure patients using the medication comprising neuregulin. The methods comprise first performing a companion diagnostic test of each patient before treatment; and then providing a suitable treatment to the patient according to the results of the companion diagnostic test. When the result of the test is within a favorite treatment zone, the patient is suitable for heart failure treatment by administering an effective amount of neuregulin.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
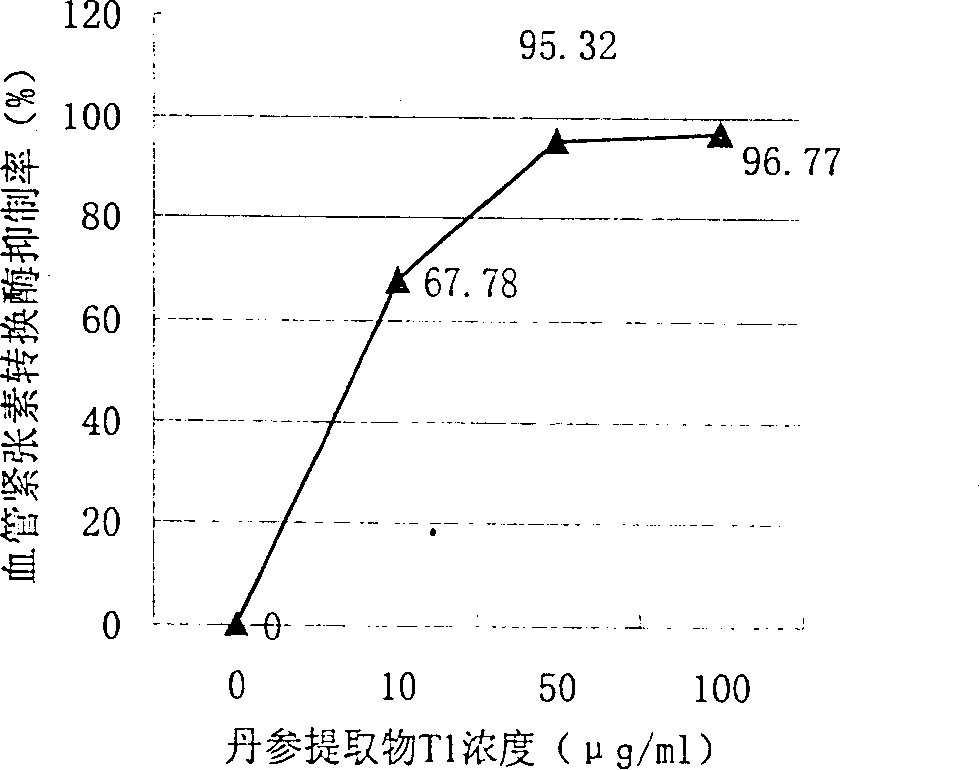
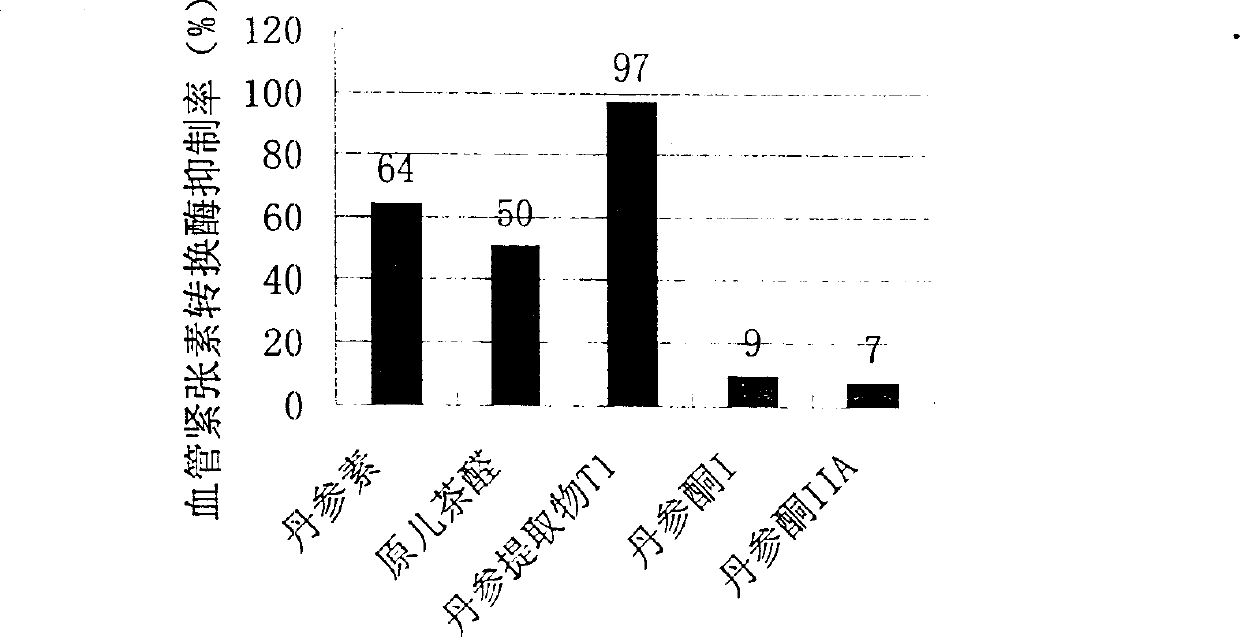
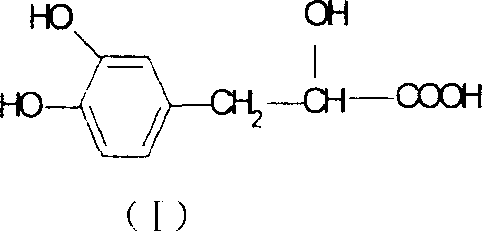
Water soluble extract of red sage root and its preeparation method and use
InactiveCN1534010AThe method is simpleGood curative effectOrganic active ingredientsMetabolism disorderSalvia miltiorrhizaNephrosis
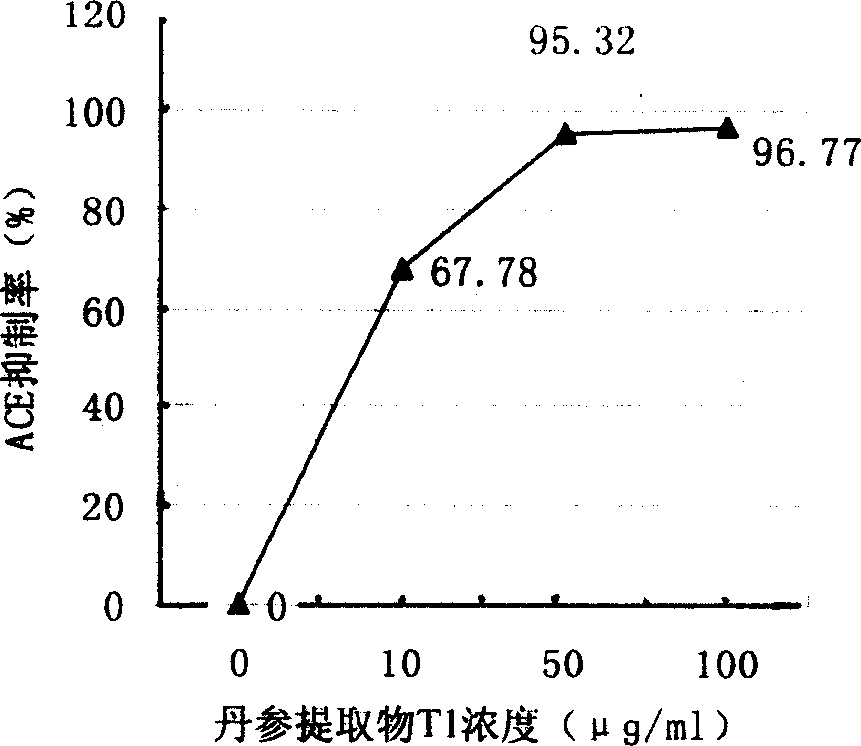
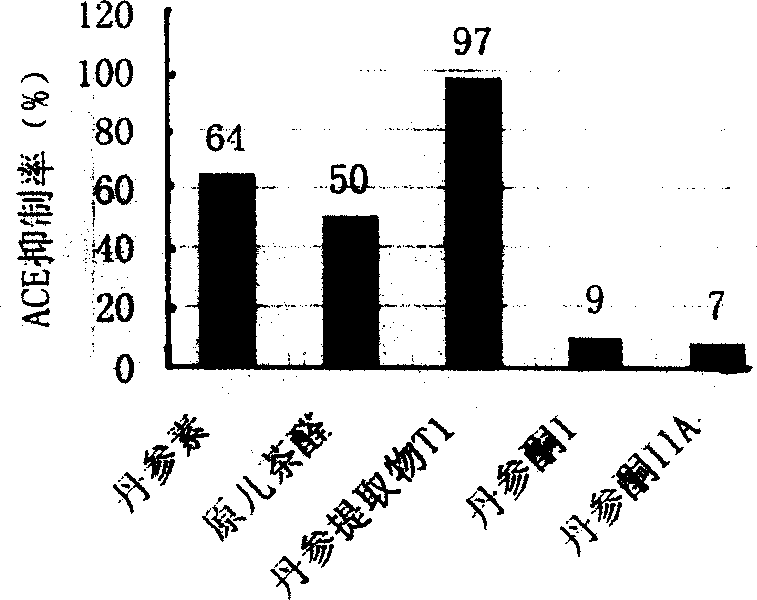
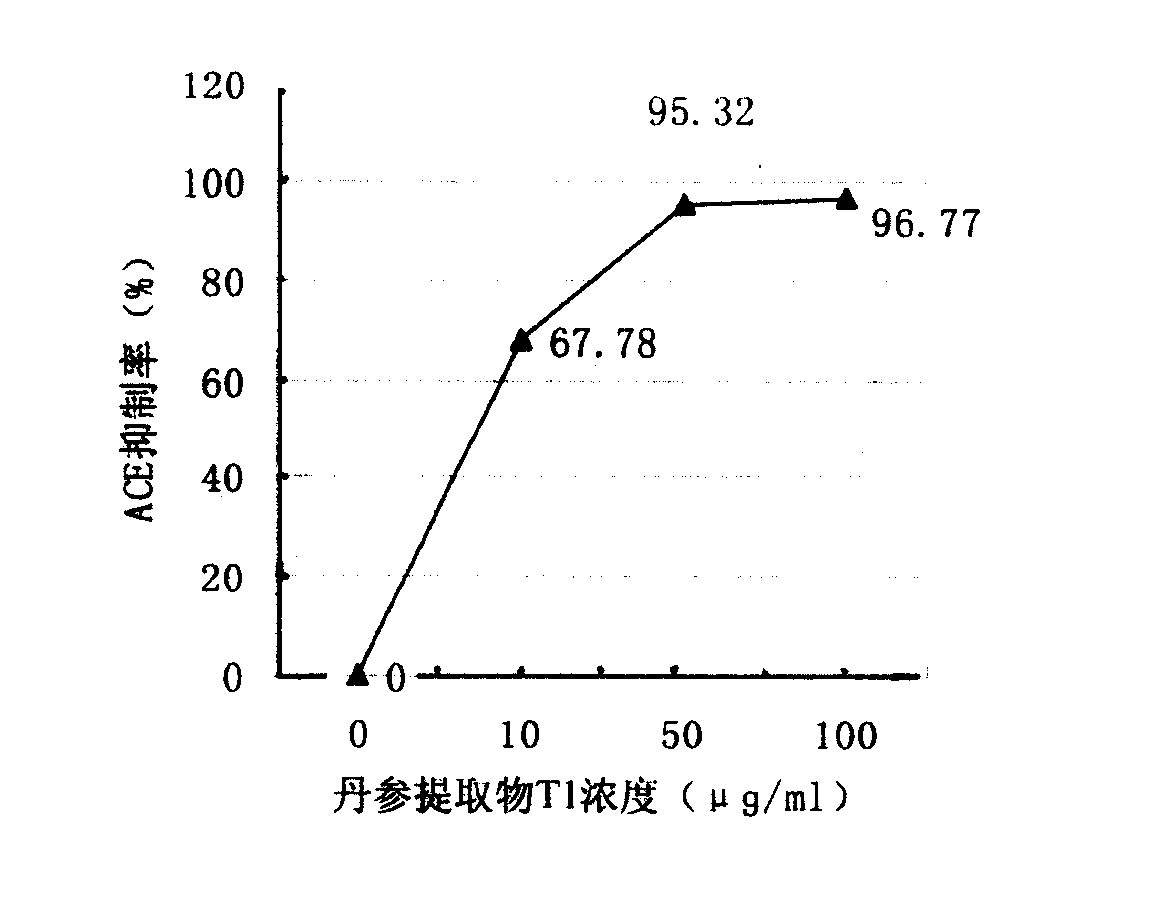
A water-soluble extract of red sage root containing danshinolic acid B, danshensu and proshikonic acid, its preparing process, and its application in preparing the angiotonin converzyme depressant medicines for treating hypertension, and hypertension accompanied by heart failure or diabetes or nephrosis are disclosed.
Owner:CHENGDU DIAO PHARMA GROUP
Docosahexaenoic acid for the treatment of heart failure
InactiveUS20120046363A1Preventing cardiac MPTP openingInhibiting cardiomyocyteBiocideAnimal repellantsCardiac failure therapyDocosahexaenoic acid
There is currently no completely effective treatment for heart failure. Considering the need for, and current void in the medical field for, a treatment for heart failure, the invention is drawn to treating heart failure. In particular aspects, the invention is drawn to the discovery that certain polyunsaturated fatty acids (PUFAs) and doses thereof are useful for treating heart failure. In other particular aspects, the invention is drawn to the discovery that certain PUFAs and doses thereof are useful for preserving mitochondrial function in a heart failure subject.
Owner:UNIV OF MARYLAND BALTIMORE
Compositions and methods for treating heart failure
ActiveUS20170326204A1Increase differentiationSimple structureNeuregulinsPeptide/protein ingredientsNeuregulinCardiac failure therapy
The present invention provides methods for treating chronic heart failure patients using the medication comprising neureplin. The methods comprise first performing a companion diagnostic test of each patient before treatment; and then providing a suitable treatment to the patient according to the results of the companion diagnostic test. When the result of the test is within a favorite treatment zone, the patient is suitable for heart failure treatment by administering an effective amount of neuregulin.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
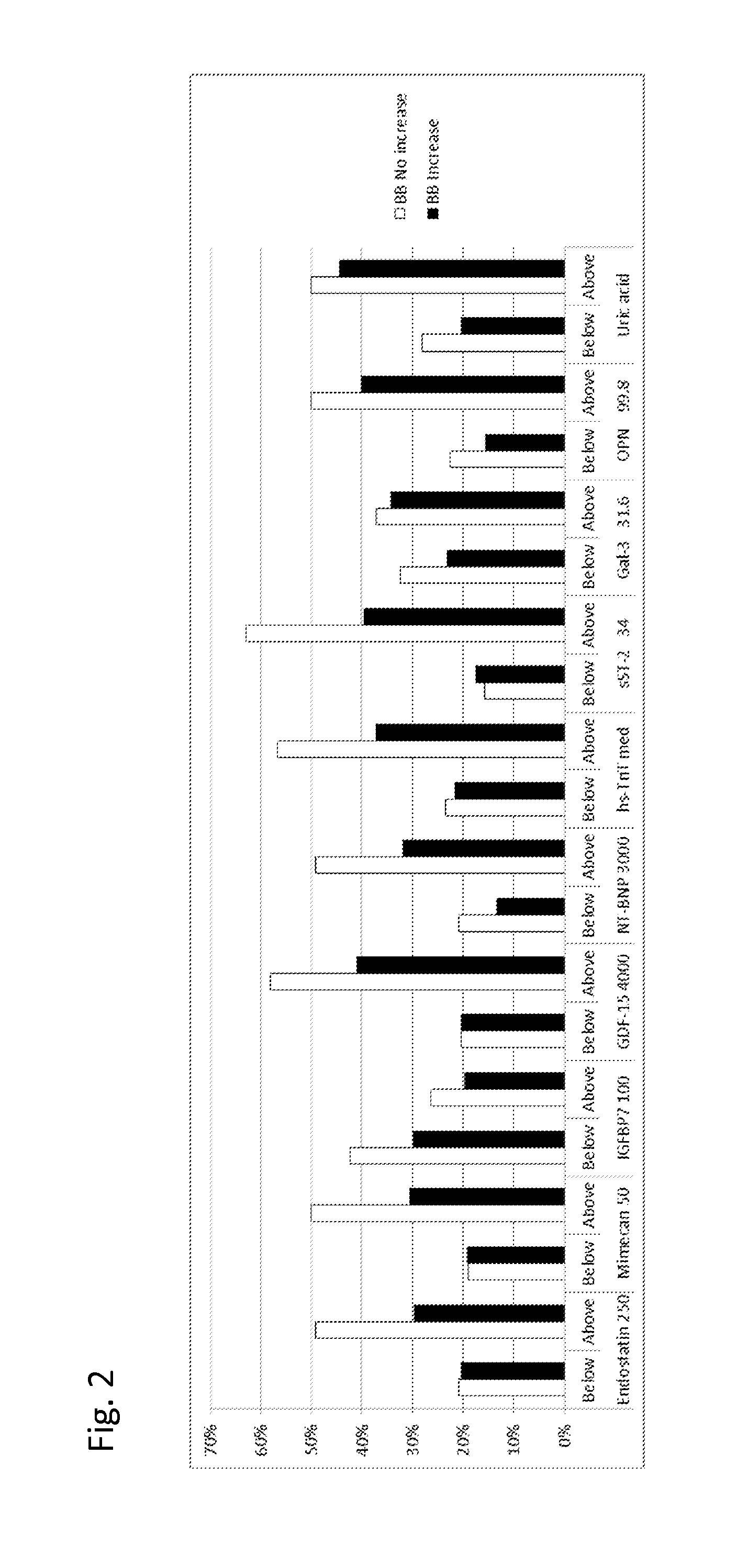
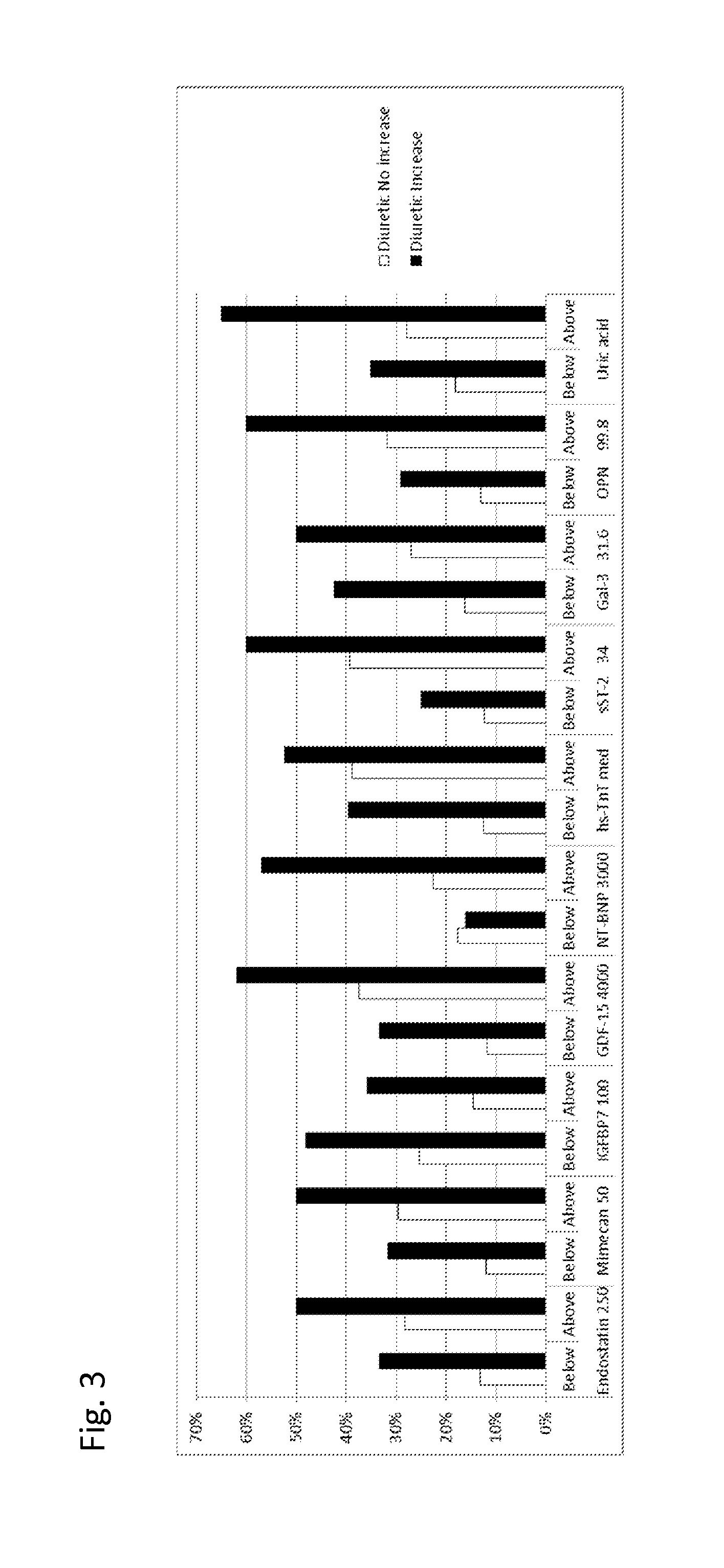
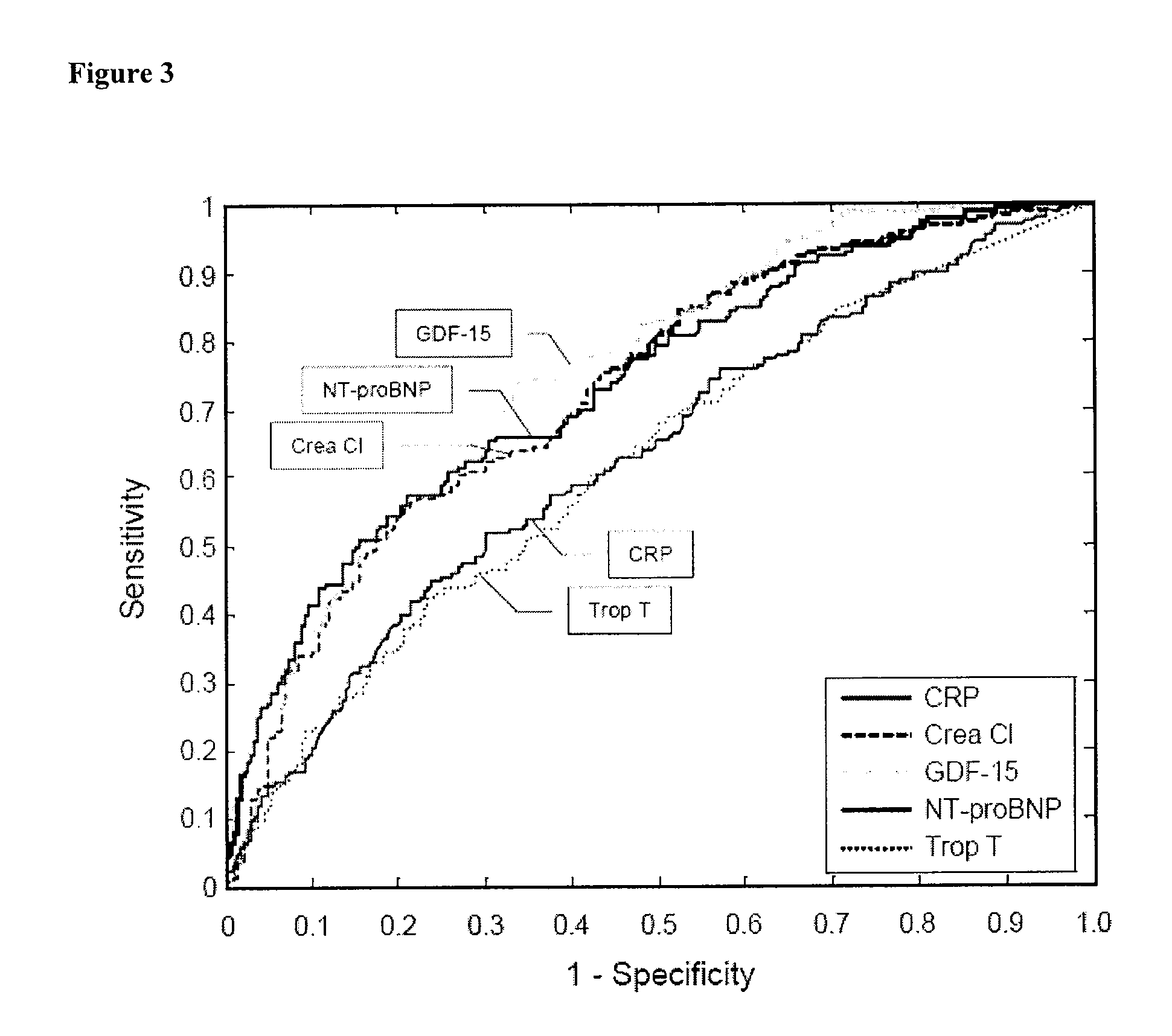
Biomarkers in the selection of therapy of heart failure
InactiveUS20150268251A1Bioreactor/fermenter combinationsBiological substance pretreatmentsBeta blockerBiomarker (petroleum)
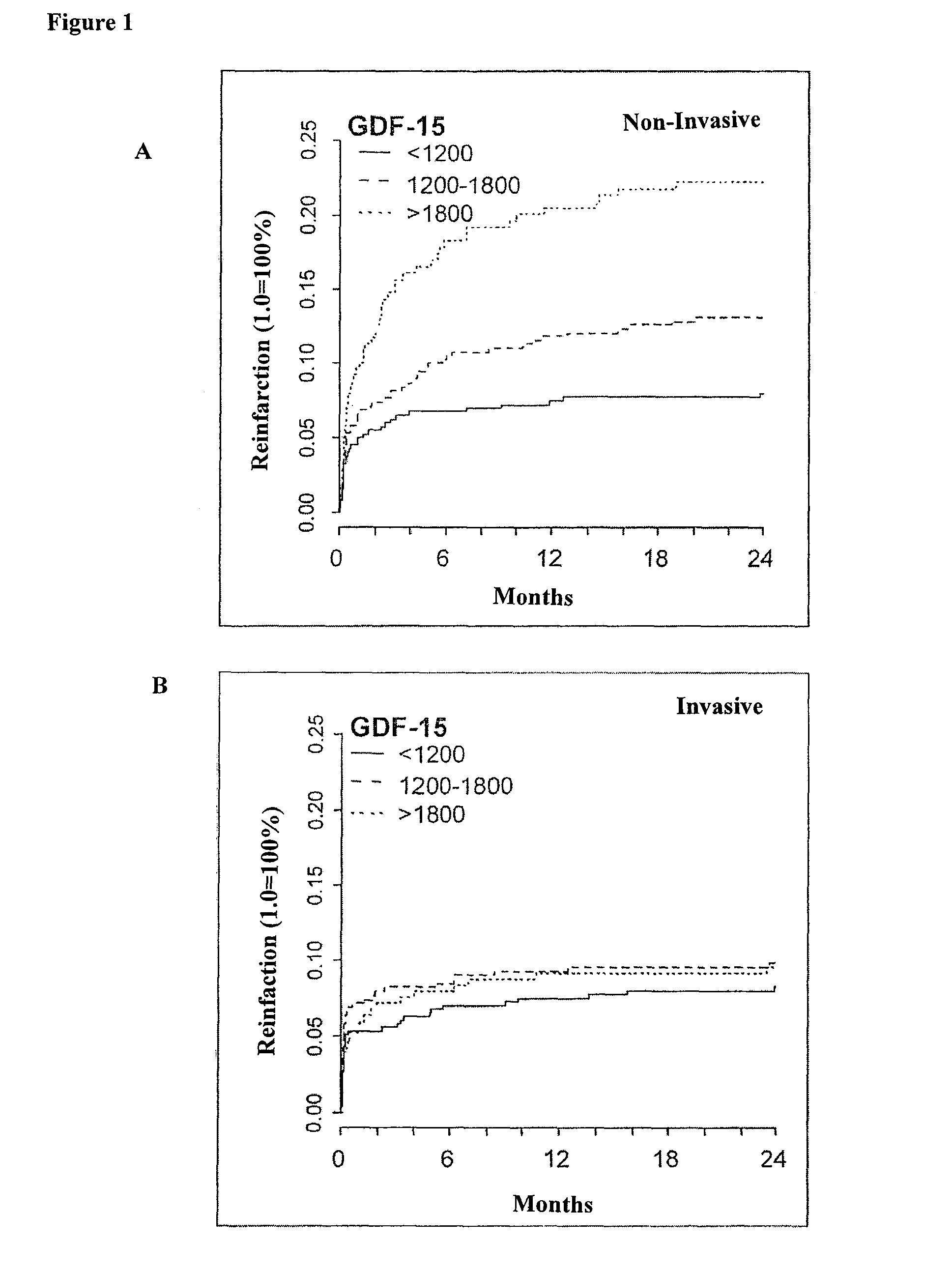
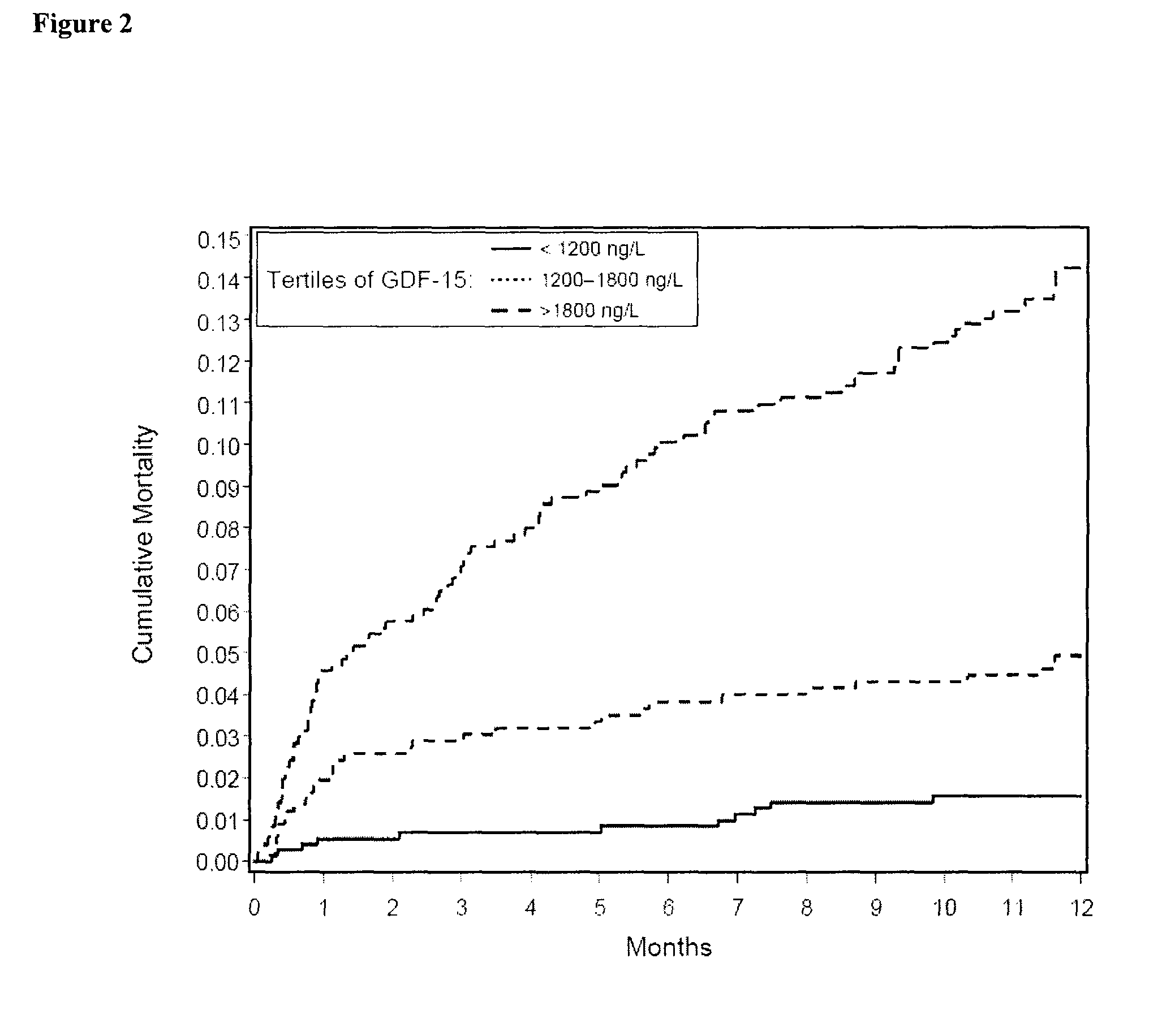
The present invention relates to a method for identifying a subject being eligible to the administration of at least one medicament selected from the group consisting of a beta blocker, an aldosterone antagonist, a diuretic, and an inhibitor of the renin-angiotensin system. The method is based on the determination of the amount of at least one biomarker selected from the group consisting of GDF-15 (Growth Differentiation Factor 15), endostatin, mimecan, IGFBP7 (IGF binding protein 7), a cardiac Troponin, a BNP-type peptide, uric acid, Gal3 (Galectin-3), osteopontin, sST2 (soluble ST2), PlGF, sFlt-1, P1NP, Cystatin C, Prealbumin, and Transferrin in a sample from a subject suffering from heart failure. Further, the method comprises the step of comparing the, thus, determined amount with a reference amount. Further envisaged by the present invention are kits and devices adapted to carry out the method of the present invention. The present invention also relates to a system for identifying a subject being eligible to the administration of at least one medicament as disclosed herein and to reagents and kits used in performing the methods as disclosed herein.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC
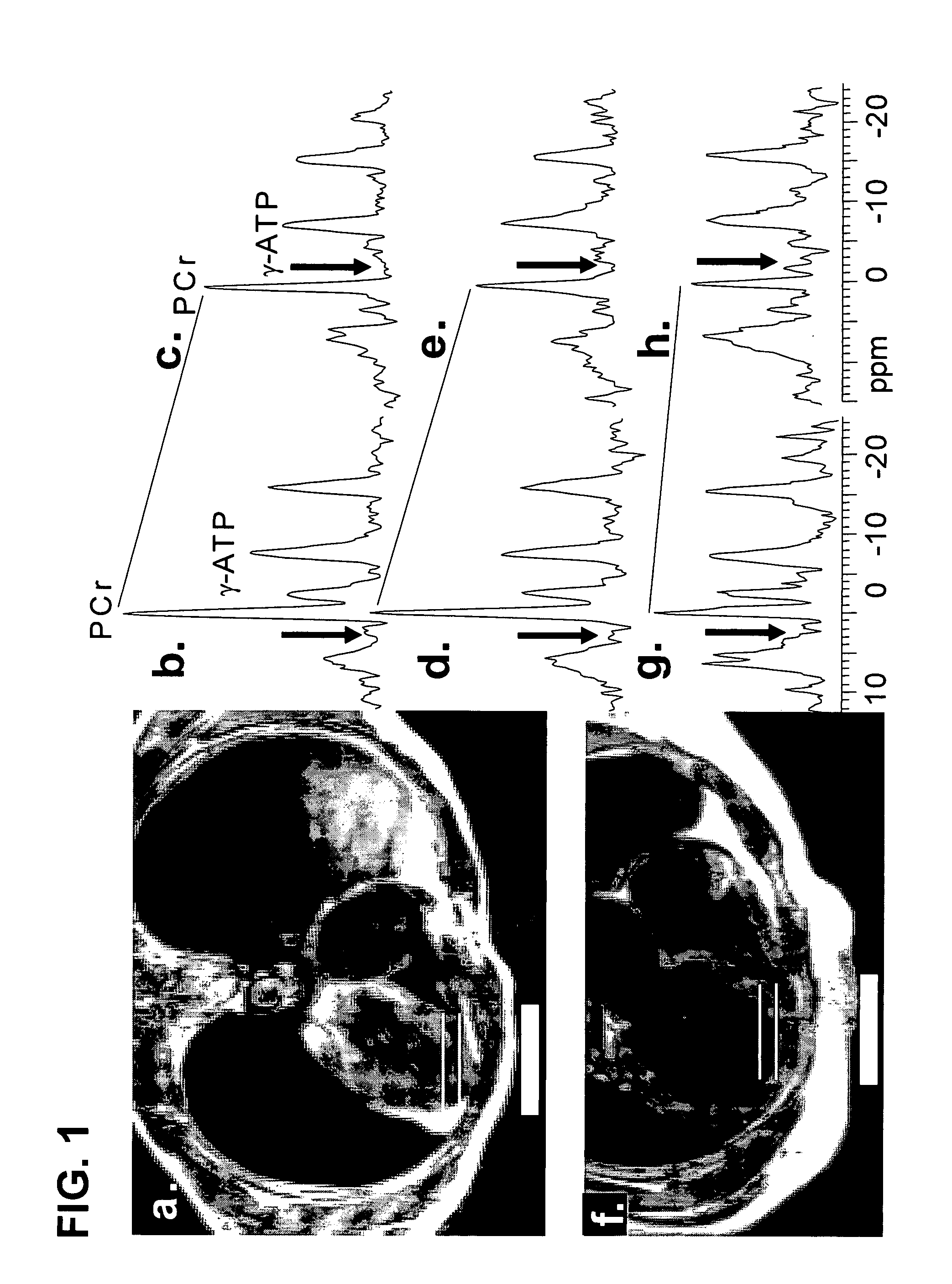
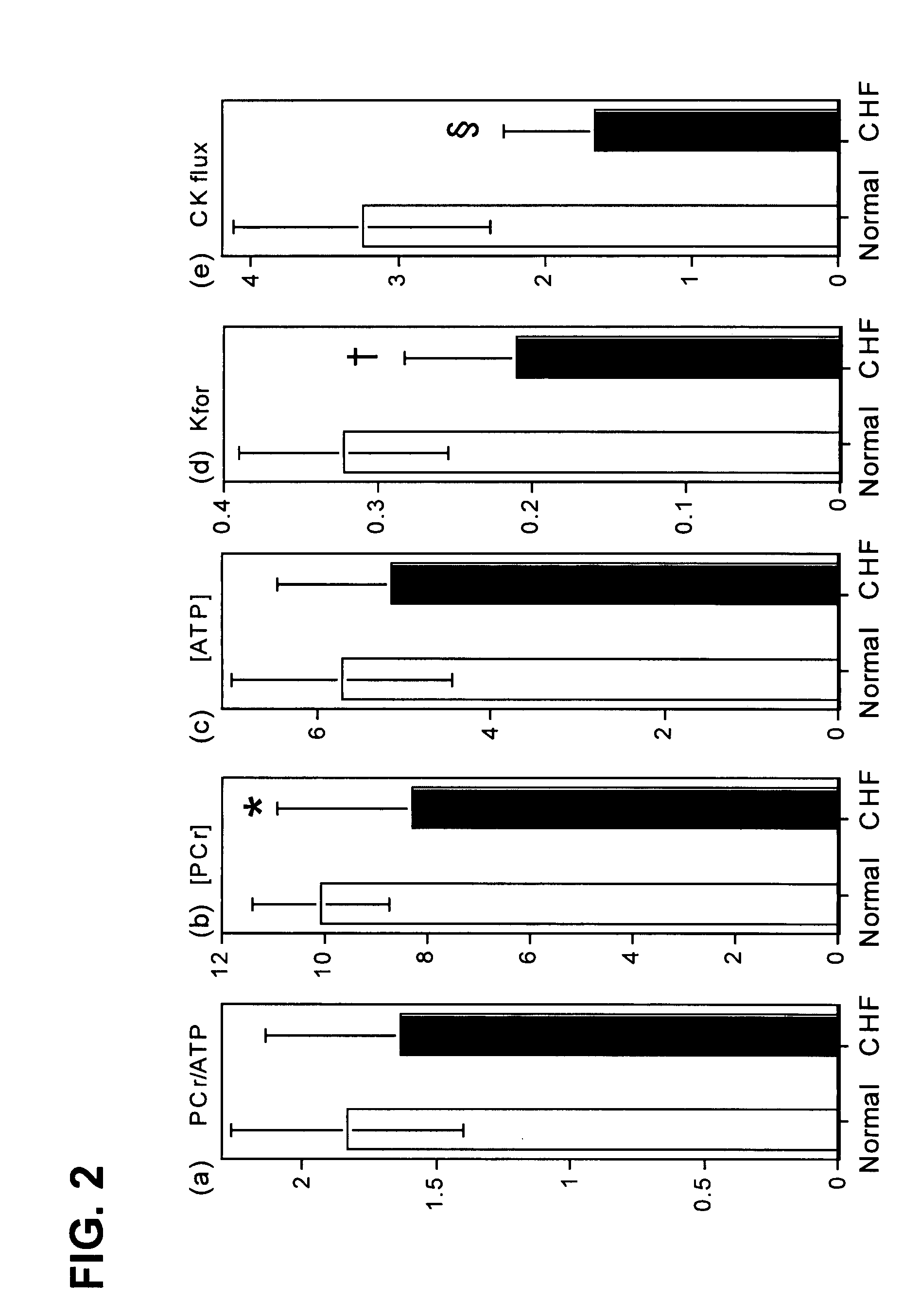
Methods to improve creatine kinase metabolism and contractile function in cardiac muscle for the treatment of heart failure
InactiveUS20070265221A1Increase supplyImproving cardiac creatine kinase metabolismCompound screeningApoptosis detectionMyocardial Creatine KinaseHuman heart
The present invention relates to novel treatments of mammalian and human heart failure directed at improving cardiac creatine kinase metabolism, the prime energy reserve of cardiac muscle. The invention also relates to novel treatments using gene transfer vectors to increase myocardial creatine kinase protein expression and / or creatine kinase activity, as well as flux through the creatine kinase reaction and to thereby improve cardiac contractile function and ameliorate remodeling in heart failure. The invention further relates to methods for screening and identifying compounds that increase creatine kinase expression and / or creatine kinase activity as potential pharmaceutical compositions for heart failure therapy.
Owner:NANOCOR THERAPEUTICS
Myoblast treatment of diseased or weakened organs
InactiveUS20070009499A1Increase blood flowImprove bindingBiocidePeptide/protein ingredientsHeterograftsWeakness
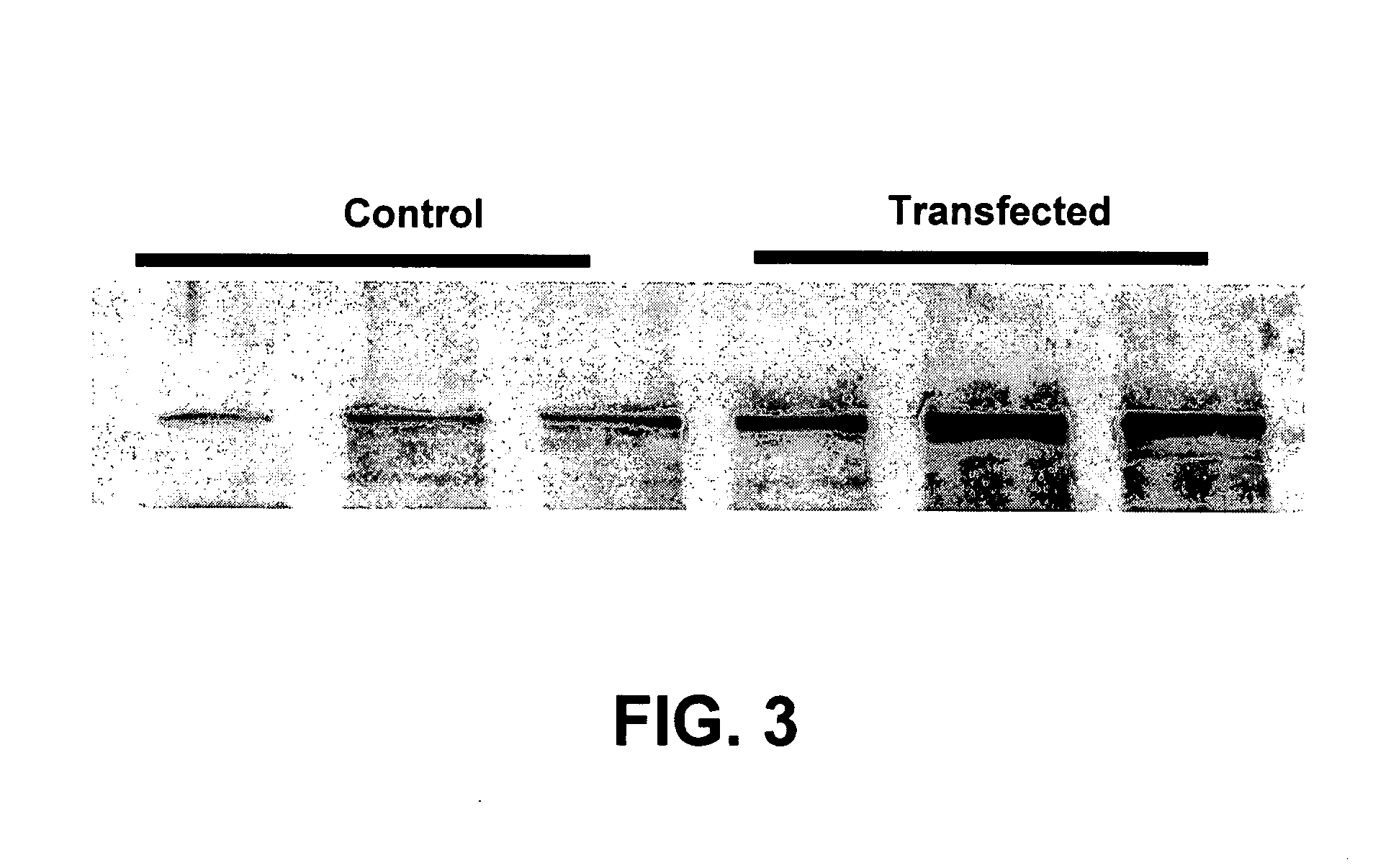
Bioengineering the regenerative heart or other body organ in need of greater muscle mass or improved blood perfusion provides a novel treatment for organ weakness or failure. In the case of cardiac failure treatment, on May 14, 2002, a 55-year-old man suffering ischemic myocardial infarction received 25 injections carrying 465 million cGMP-produced pure myoblasts into his myocardium after coronary artery bypass grafting. Three myogenesis mechanisms were elucidated with 17 human / porcine xenografts using cyclosporine as immunosuppressant. Some myoblasts developed to become cardiomyocytes. Others transferred their nuclei into host cardiomyocytes through natural cell fusion. As yet others formed skeletal myofibers with satellite cells. De novo production of contractile filaments augmented heart contractility. Human myoblasts transduced with VEGF165 gene produced six times more capillaries in porcine myocardium than placebo. Xenograft rejection was not observed for up to 20 weeks despite cyclosporine discontinuation at 6 weeks.
Owner:LAW PETER
Heart failure treatment device and method
A method and apparatus for treating heart failure is configured to be placed about at least a portion of a patient's heart to apply a mild compressive force on the heart over a range of elastic deformation of the apparatus. The apparatus can be shifted to second range of deformation. In some embodiments, the apparatus is shifted to the second range of deformation by application of a stimulus or alteration of environmental conditions beyond a threshold level.
Owner:PARACOR MEDICAL INC
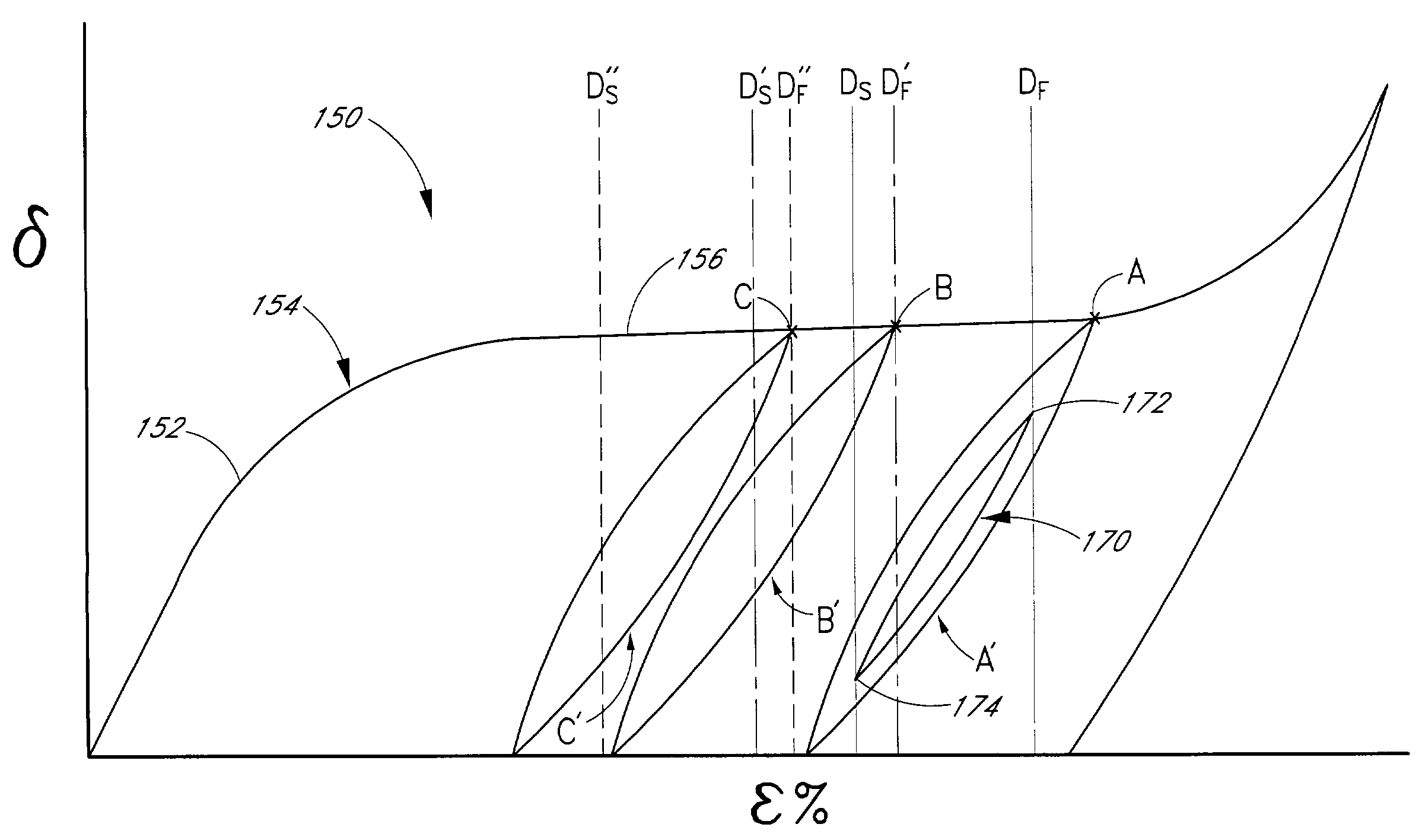
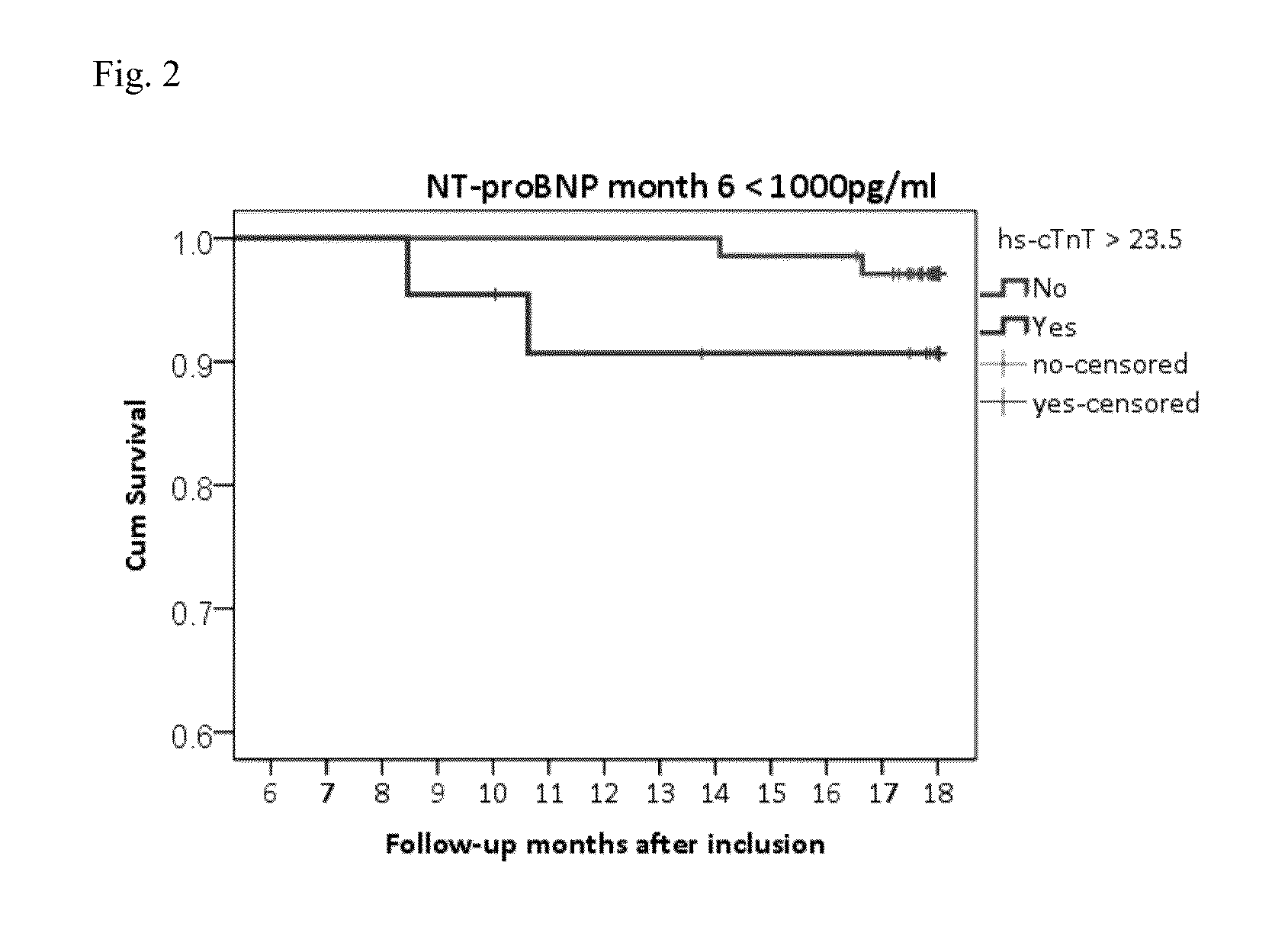
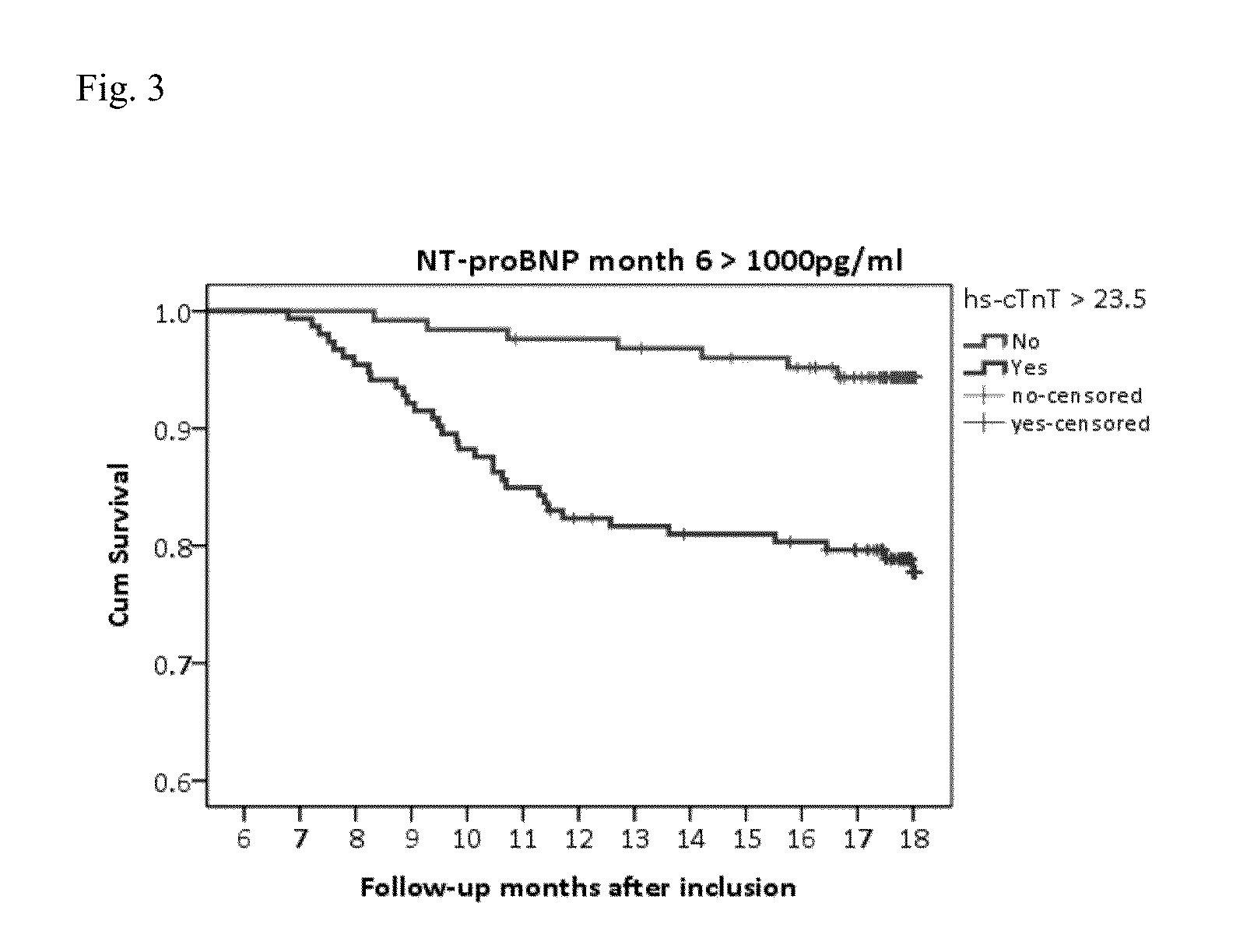
NTproBNP AND cTnT BASED THERAPY GUIDANCE IN HEART FAILURE
The present invention relates to a method for guiding heart failure treatment in a subject suffering from heart failure. The method is based on the determination of the amount of a BNP-type peptide and a cardiac troponin in a sample from said subject. Further envisaged by the present invention are kits and devices adapted to carry out the present invention. The present invention also relates to a system for guiding heart failure treatment in a subject suffering from heart failure as disclosed herein and to reagents and kits used in performing the methods disclosed herein.
Owner:ROCHE DIAGNOSTICS OPERATIONS INC +1
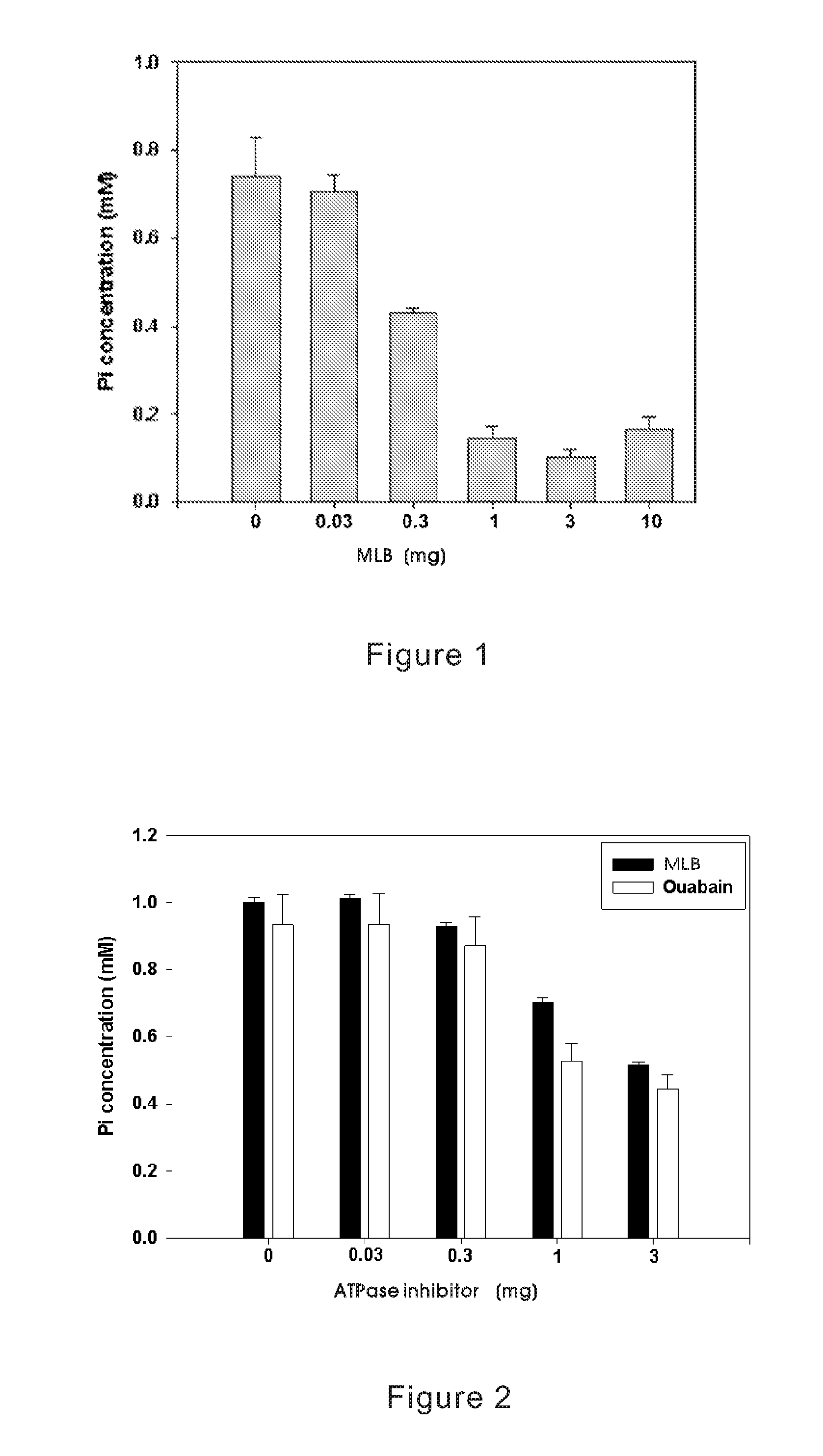
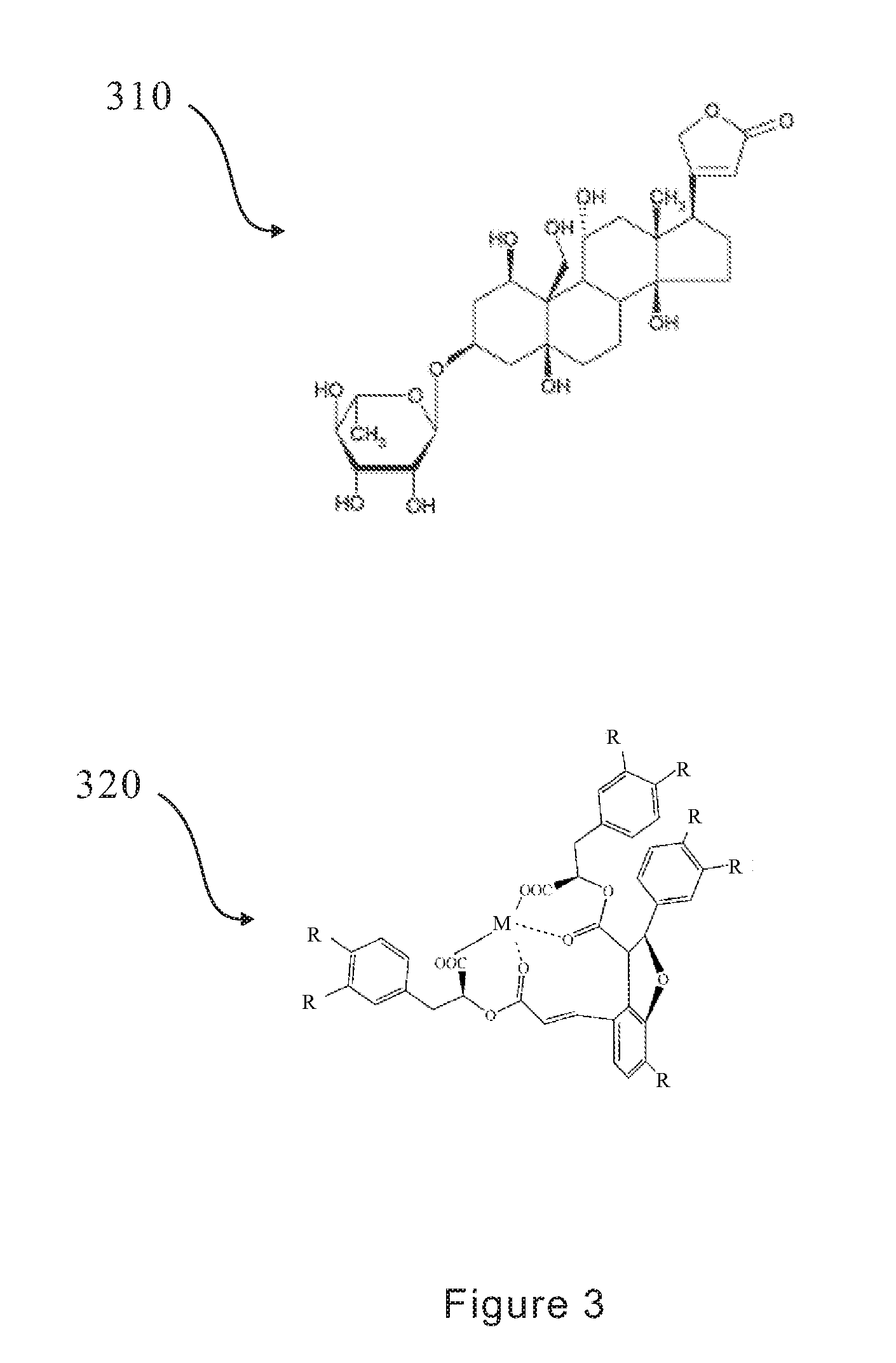
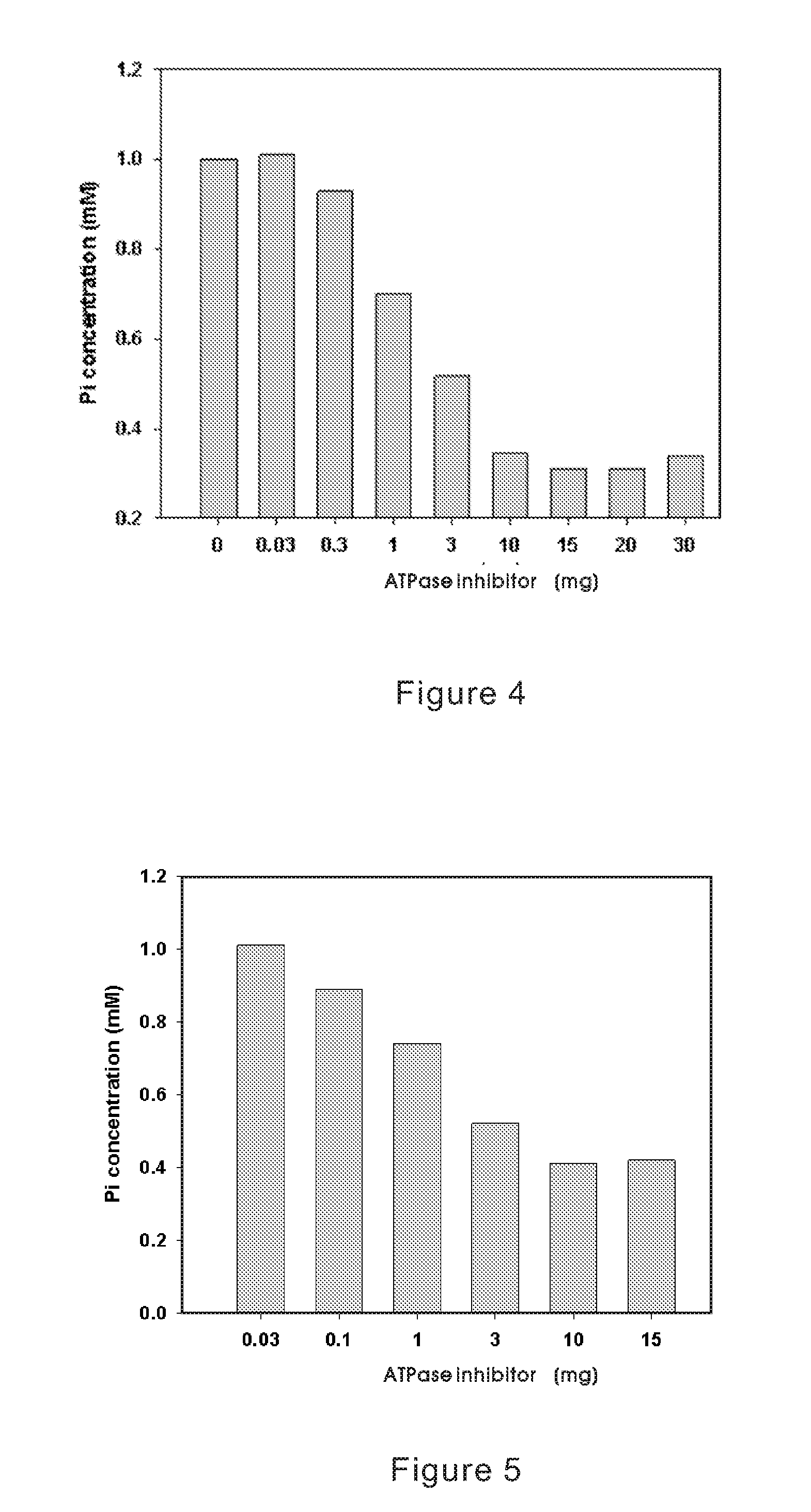
Method for Inhibiting Cellular Na+-K+ ATPase Activity
InactiveUS20070293462A1Inhibiting ATPase activitySatisfies needBiocideOrganic active ingredientsPotassiumMagnesium lithospermate B
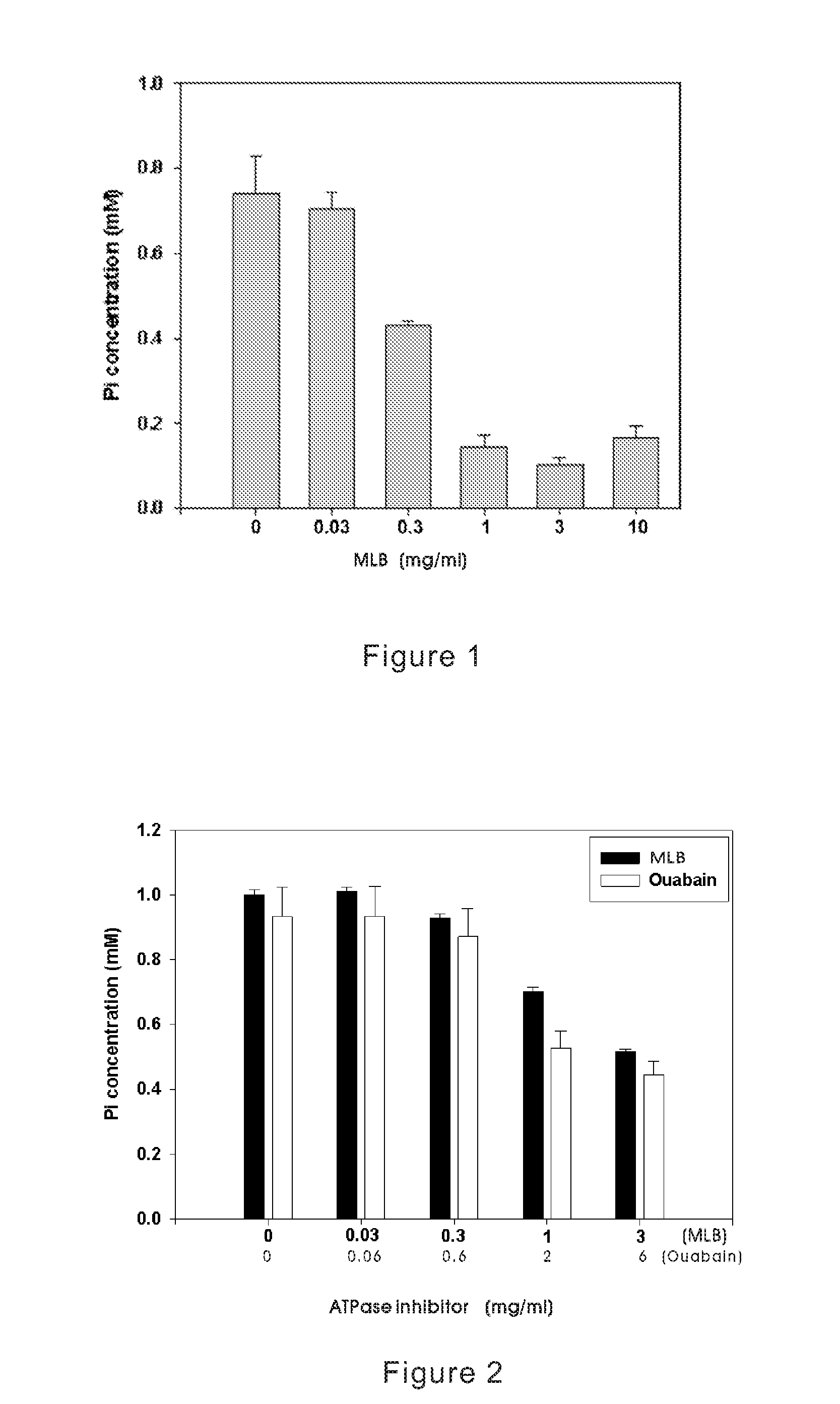
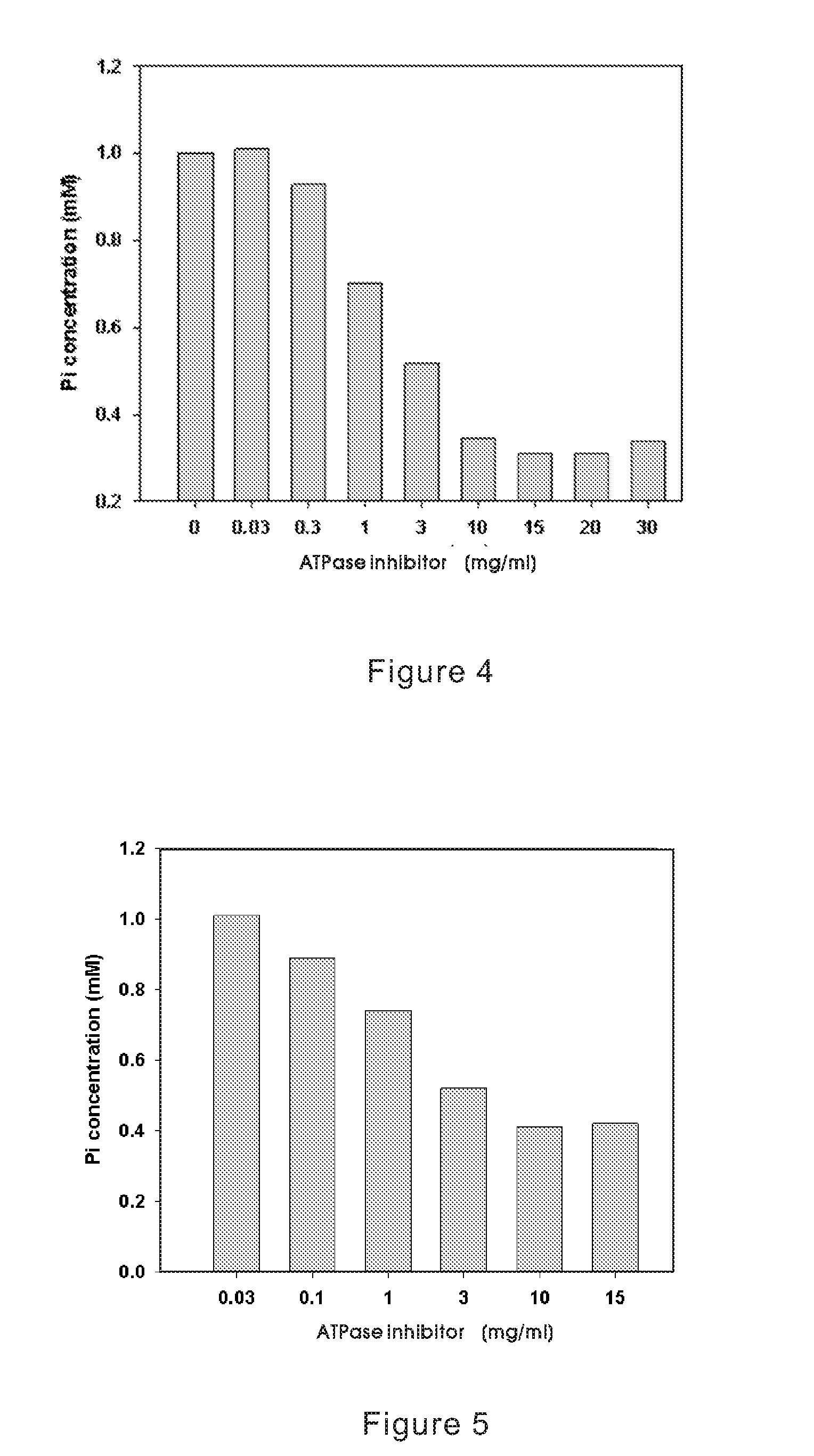
The present invention discloses an inhibitive effect of Na+-K+-ATPase caused by a compound selected from the group consisting of magnesium lithospermate B (MLB), isomer, prodrug, derivative, pharmaceutically acceptable salt, and a composition thereof. In this invention, the variations of Na+-K+-ATPase activity were monitored with increasing MLB concentrations, and the result shows the Na+-K+ ATPase activity is repressed by MLB. An outcome of the inhibitory effect, the function of cellular sodium / potassium lo exchanger is reduced and cellular calcium ion concentration is increased. That is, the MLB is useful for inhibiting the function of cellular Na+-K+ pump, and further brings the utility for cardiac stimulation, diuretic enhancement, heart failure curing, and so on.
Owner:JASON LIFE TECH
Heart failure treatment
InactiveUS20160082084A1Preventing heart failurePeptide/protein ingredientsPharmaceutical delivery mechanismHeart failure cellCardiac failure therapy
The present invention relates to treating and / or preventing heart failure or one or more individual heart failure phenotypes in mammals using placental growth factor 2 (PlGF-2).
Owner:COBIORES NV
Method for Inhibiting Cellular Na+-K+ ATPase Activity
InactiveUS20070293459A1Meet actual needsSignificant positive effectOrganic active ingredientsBiocideApoptosisPotassium
The present invention discloses an inhibitive effect of Na+—K+-ATPase caused by a compound selected from the group consisting of magnesium lithospermate B (MLB), isomer, prodrug, derivative, pharmaceutically acceptable salt, and a composition thereof. In this invention, the variations of Na+—K+-ATPase activity were monitored with increasing MLB concentrations, and the result shows the Na+—K+ ATPase activity is repressed by MLB. An outcome of the inhibitory effect, the function of cellular sodium / potassium exchanger is reduced and cellular calcium ion concentration is increased. The cerebral ischemia test exhibited MLB provides an effective repression of cell infarct. That is, the MLB is useful for inhibiting the function of cellular Na+—K+ pump, and further brings the utility for cardiac stimulation, diuretic enhancement, heart failure curing, anti-anoxia, neurocyte apoptosis protection, apoplexy prevention and treatment, and so on.
Owner:JASON LIFE TECH
Pharmaceutical composition for treating heart failure and preparation method thereof
ActiveCN108310207APrecise recipeEasy to useCardiovascular disorderPlant ingredientsCardiac failure therapyOyster
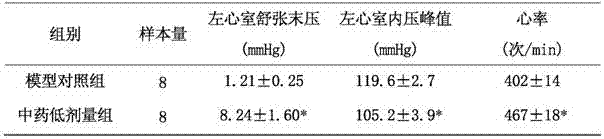
The invention provides a pharmaceutical composition for treating heart failure. The pharmaceutical composition is prepared from 12-18 parts by weight of radix bupleuri, 16-24 parts by weight of oyster, 16-24 parts by weight of radix angelicae sinensis, 40-60 parts by weight of spina date seed, 24-36 parts by weight of cortex acanthopanacis giraldii, 16-24 parts by weight of raw sun ginseng, 8-12 parts by weight of cassia bark and 12-18 parts by weight of radix polygonati officinalis. The invention also provides a preparation method and use of the pharmaceutical composition. The pharmaceuticalcomposition has reasonable compatibility and good therapeutic effects on various heart failures, has good safety and provides a novel clinical drug.
Owner:TEACHING HOSPITAL OF CHENGDU UNIV OF T C M
Medicine composition for treating congestive heart failure and application thereof
InactiveCN106860730ARemissionReduce manufacturing costCardiovascular disorderPlant ingredientsCardiac failure therapyMortality rate
The invention discloses a medicine composition for treating congestive heart failure and application thereof. The medicine composition is prepared from supernate obtained through treating the following traditional Chinese medicinal materials by a water extraction and alcohol sedimentation method: 9 to 15 parts of lineate supplejack leaves, 15 to 22 parts of black beans, 4 to 8 parts of bitter citrus immature flowers, 27 to 33 parts of millettia specisoa champ roots, 4 to 8 parts of spinach fruits, 12 to 18 parts of radices hemerocallis, 9 to 15 parts of barbate cyclea roots or stems, 12 to 18 parts of dry lotus leaves and 15 to 22 parts of wideleaf osbeckia roots. When the medicine composition is used as the medicine for treating the congestive heart failure, the medicine effect is comprehensive; the illness state of patients with congestive heart failure can be fast relieved; the heart failure death rate is reduced.
Owner:QINGDAO MUNICIPAL HOSPITAL
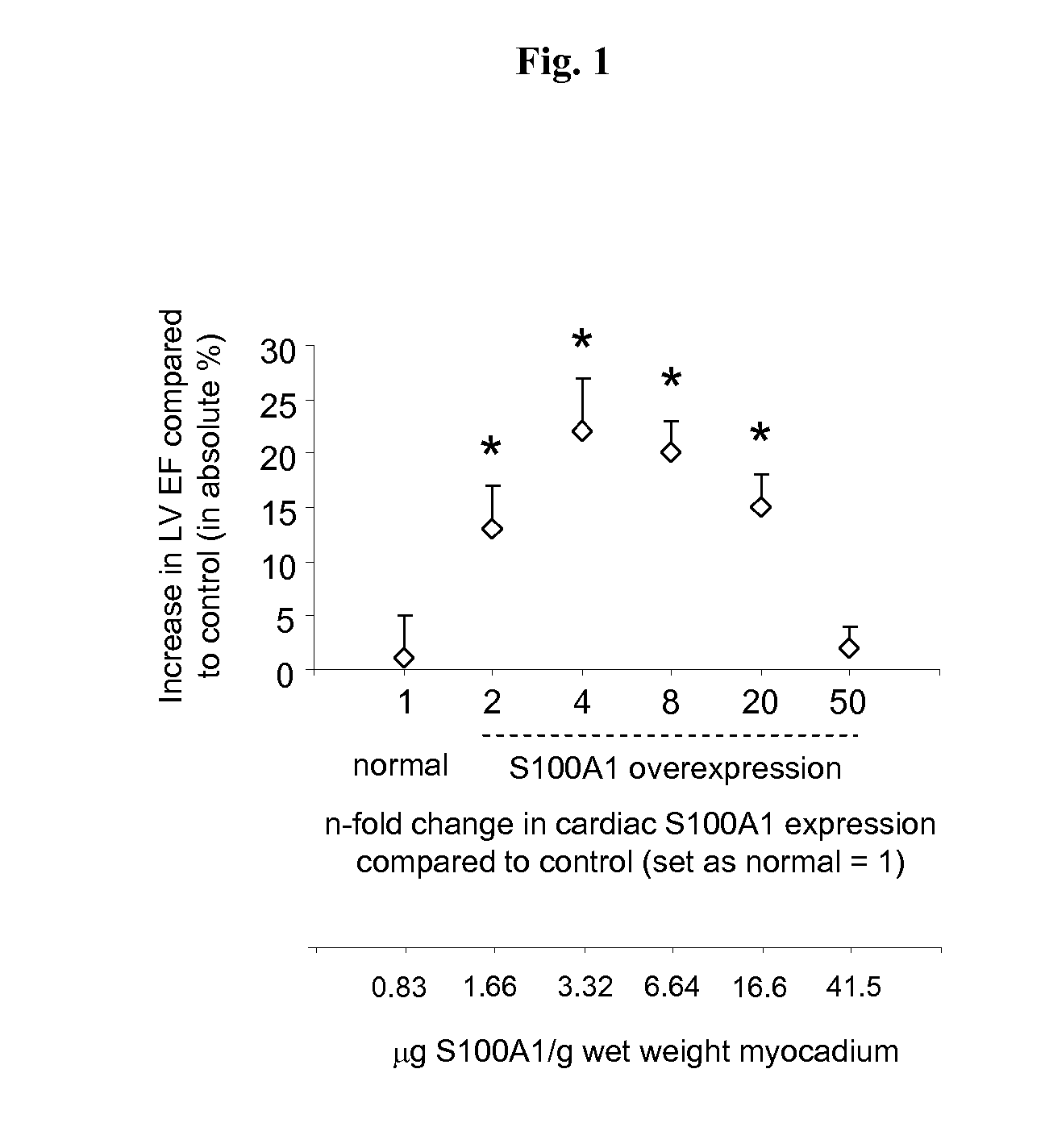
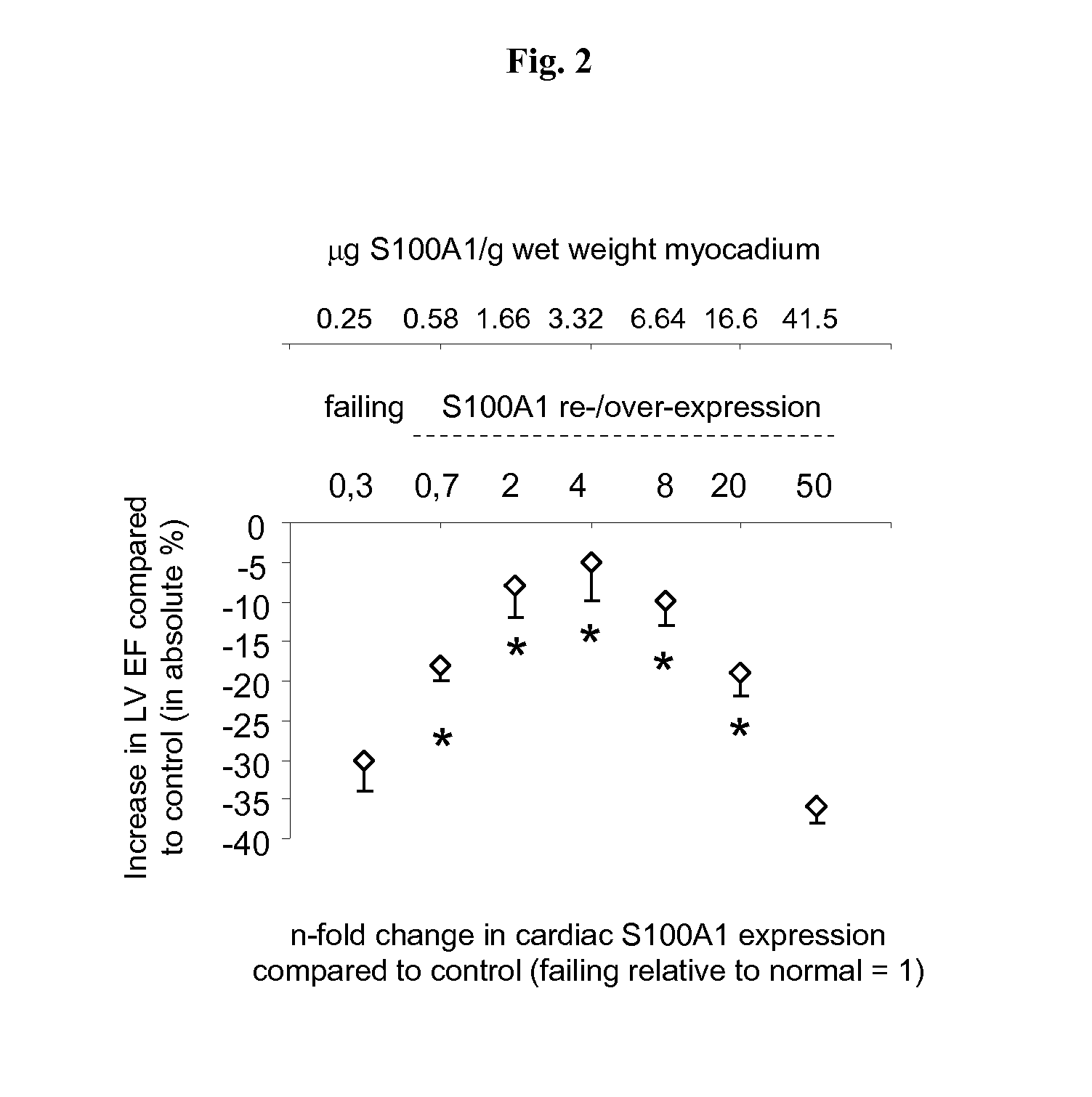
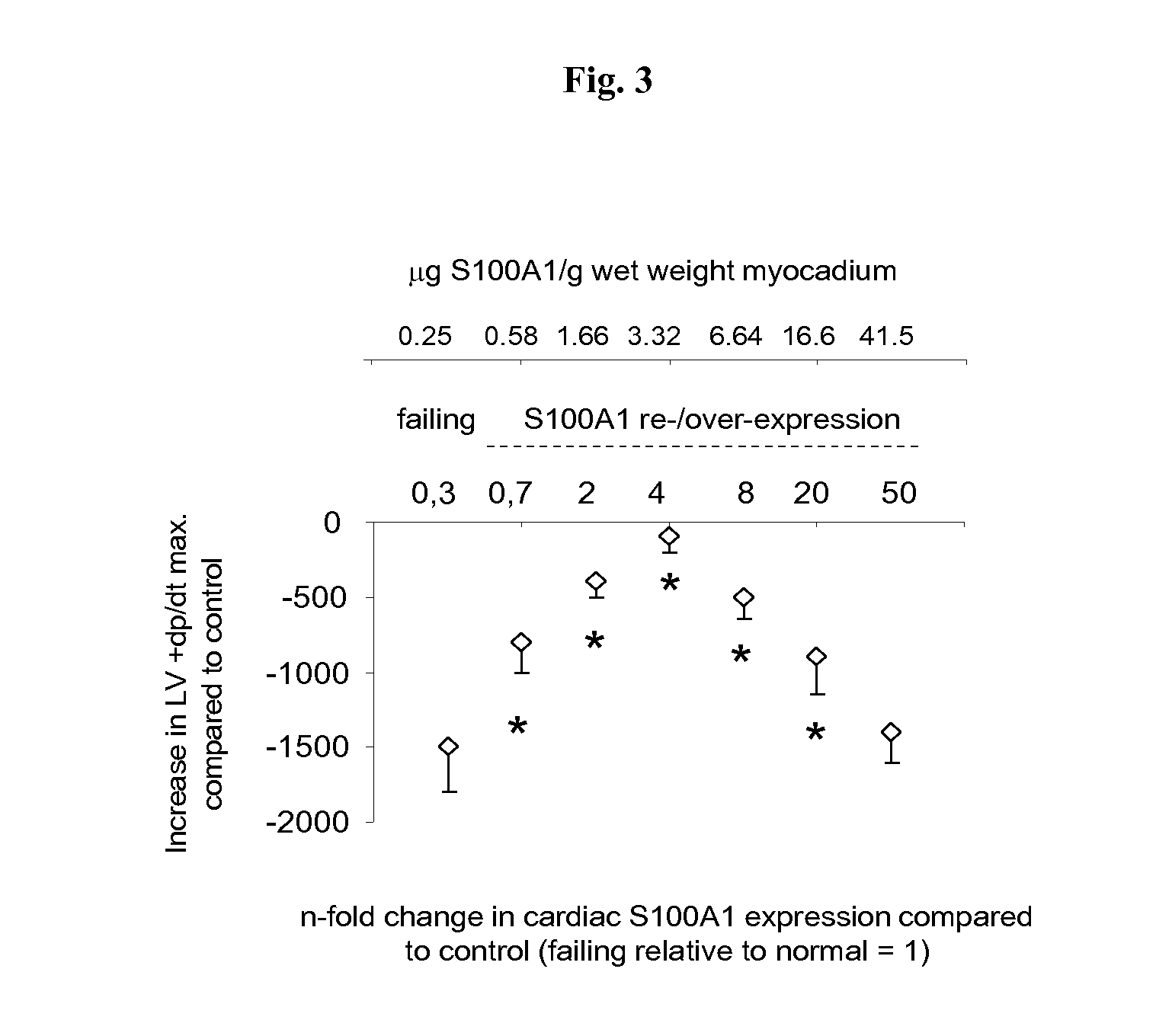
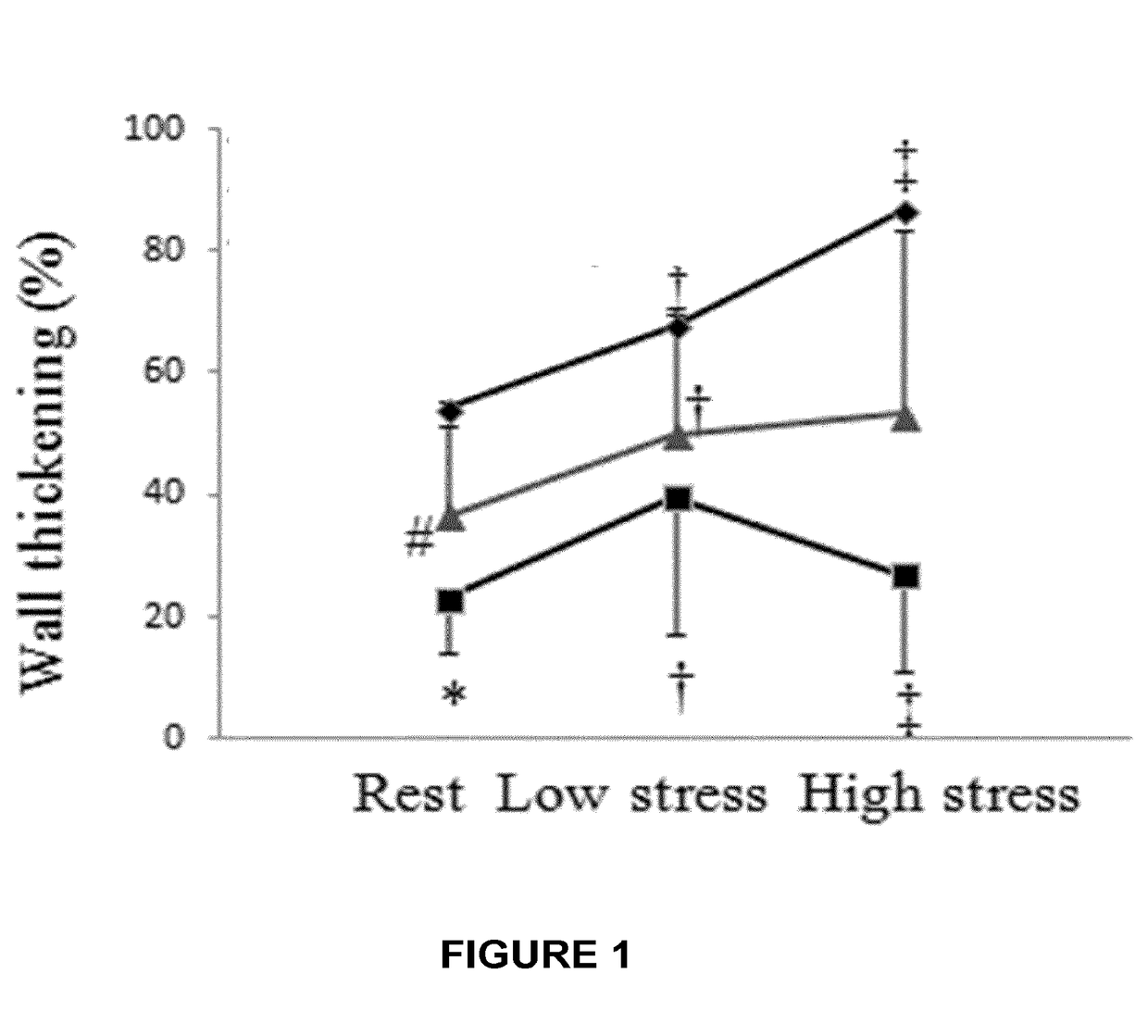
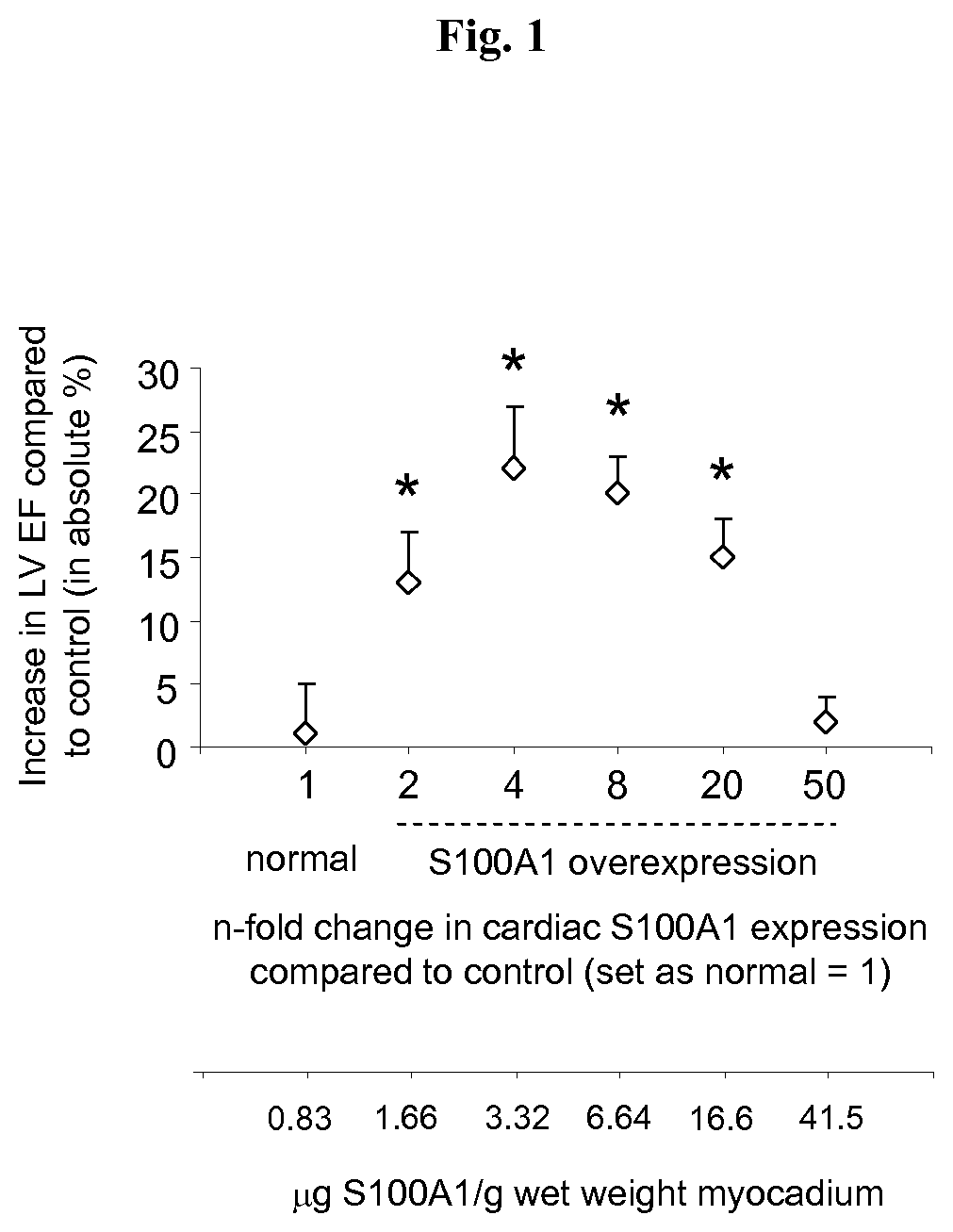
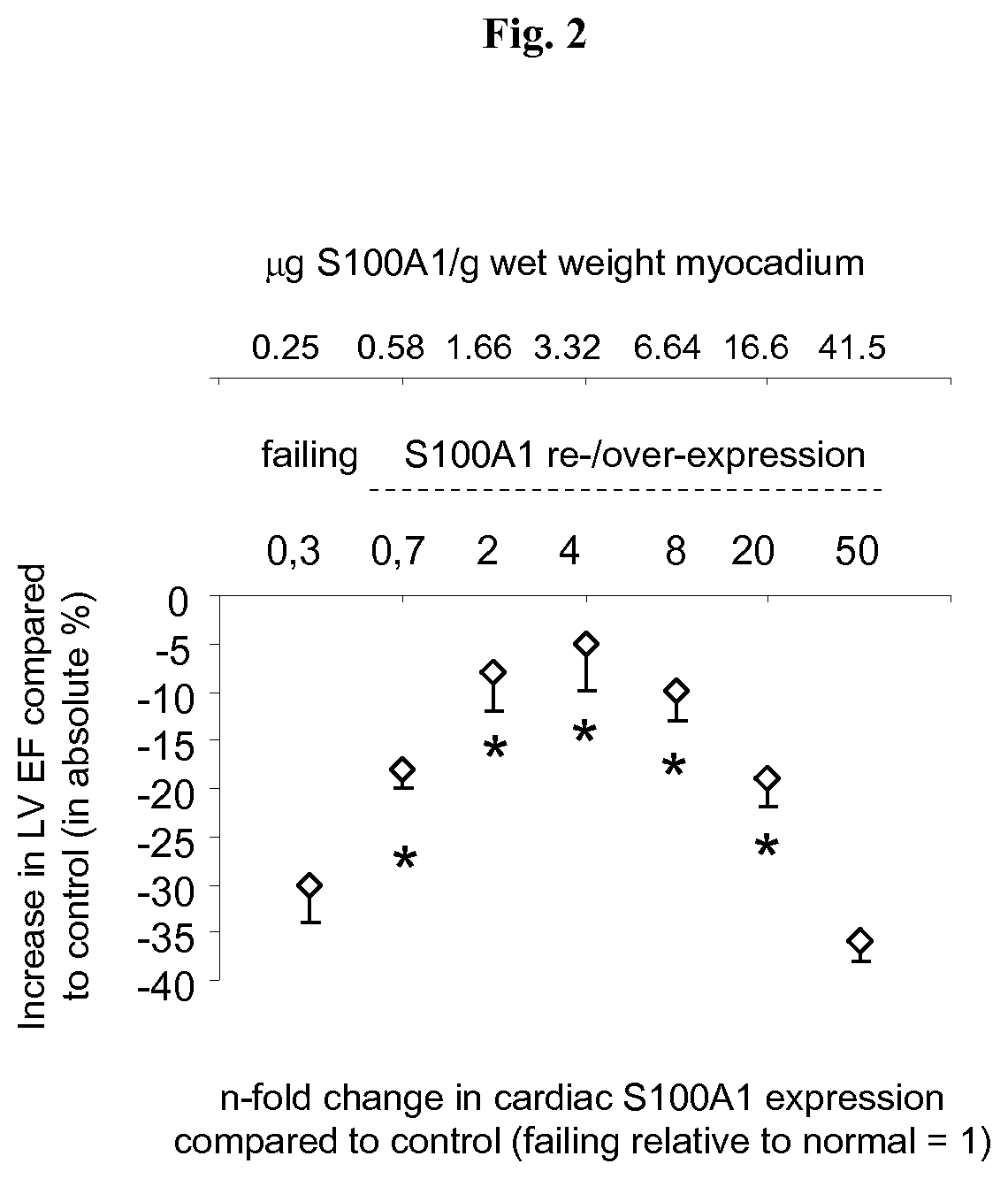
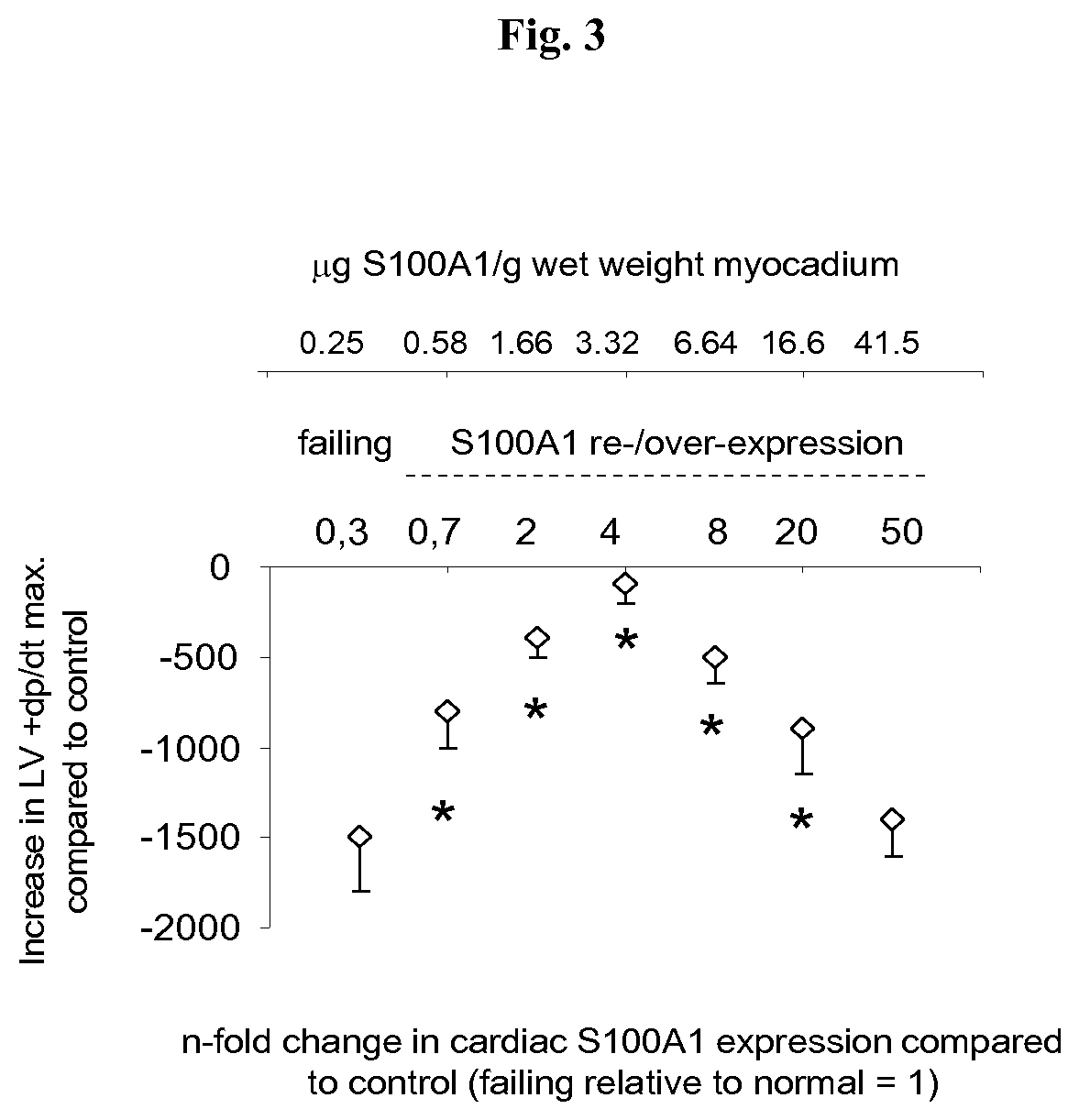
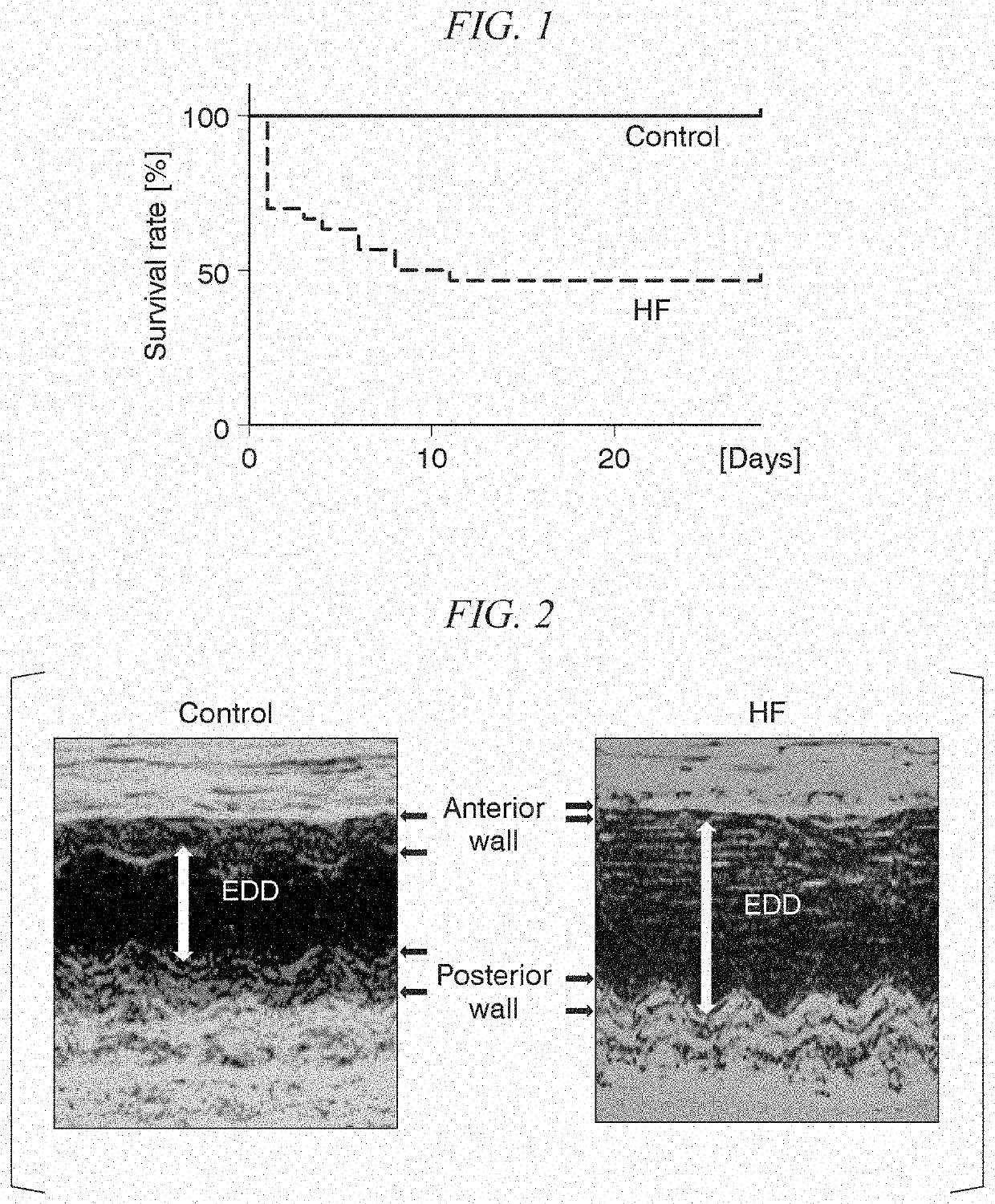
S100 BASED TREATMENT OF CARDIAC POWER FAlLURE
ActiveUS20160228504A1Enhancing cardiac powerImprove concentrationPeptide/protein ingredientsAnimals/human peptidesCardiac failure therapyS100 protein
Owner:UNIVERSITY OF HEIDELBERG
Method and a catheter device for the dynamic regulation of the venous return to the heart for the treatment of patients with heart failure
ActiveUS8668669B2Increase flow rateLoad accuratelySurgeryMedical devicesLeft subclavian veinRight atrium
Owner:HERRERA JOSE E
Heart failure treatment
InactiveUS20170319656A1Peptide/protein ingredientsPharmaceutical delivery mechanismCardiac failure therapyHeart failure cell
Owner:COBIORES NV
Neuregulin based methods for treating heart failure
ActiveUS11246909B2Increase differentiationSimple structurePeptide/protein ingredientsAntipyreticNeuregulinCardiac failure therapy
The present invention features methods of treating patients with chronic heart failure by administering a neuregulin polypeptide within a dosage range which is both effective and safe.
Owner:ZENSUN (SHANGHAI) SCI & TECH CO LTD
Assessing susceptibility to cardiac intervention, susceptibility to therapy for heart failure, risk of mortality or further cardiovascular events, and risk of subsequent pulmonary embolism in relevant patients based on determinations of GDF-15, natriuretic peptide, cardiac troponin or combinations thereof
The present invention relates to a method of identifying a subject being susceptible to a cardiac intervention based on the determination of GDF-15 in a sample of a subject in need of a cardiac intervention. Moreover, the present invention pertains to a method for predicting the risk of mortality or a further acute cardiovascular event for a subject suffering from a cardiovascular complication based on the determination of GDF-15 and a natriuretic peptide and / or a cardiac troponin in a sample the said subject. Also encompassed by the present invention are devices and kits for carrying out the aforementioned methods.
Owner:MEDIZINISCHE HOCHSCHULE HANNOVER
Water soluble extract of red sage root and its preparation method and use
A water-soluble extract of red sage root containing danshinolic acid B, danshensu and proshikonic acid, its preparing process, and its application in preparing the angiotonin converzyme depressant medicines for treating hypertension, and hypertension accompanied by heart failure or diabetes or nephrosis are disclosed.
Owner:CHENGDU DIAO PHARMA GROUP
S100 based treatment of cardiac power failure
ActiveUS10792329B2Enhancing cardiac powerImprove concentrationPeptide/protein ingredientsAnimals/human peptidesCardiac failure therapyPharmaceutical drug
Owner:UNIVERSITY OF HEIDELBERG
Method of evaluating pathological conditions of heart failure
PendingUS20210349079A1Prevention of failureTreatment of failureOrganic active ingredientsDisease diagnosisPeripheral blood mononuclear cellEngineering
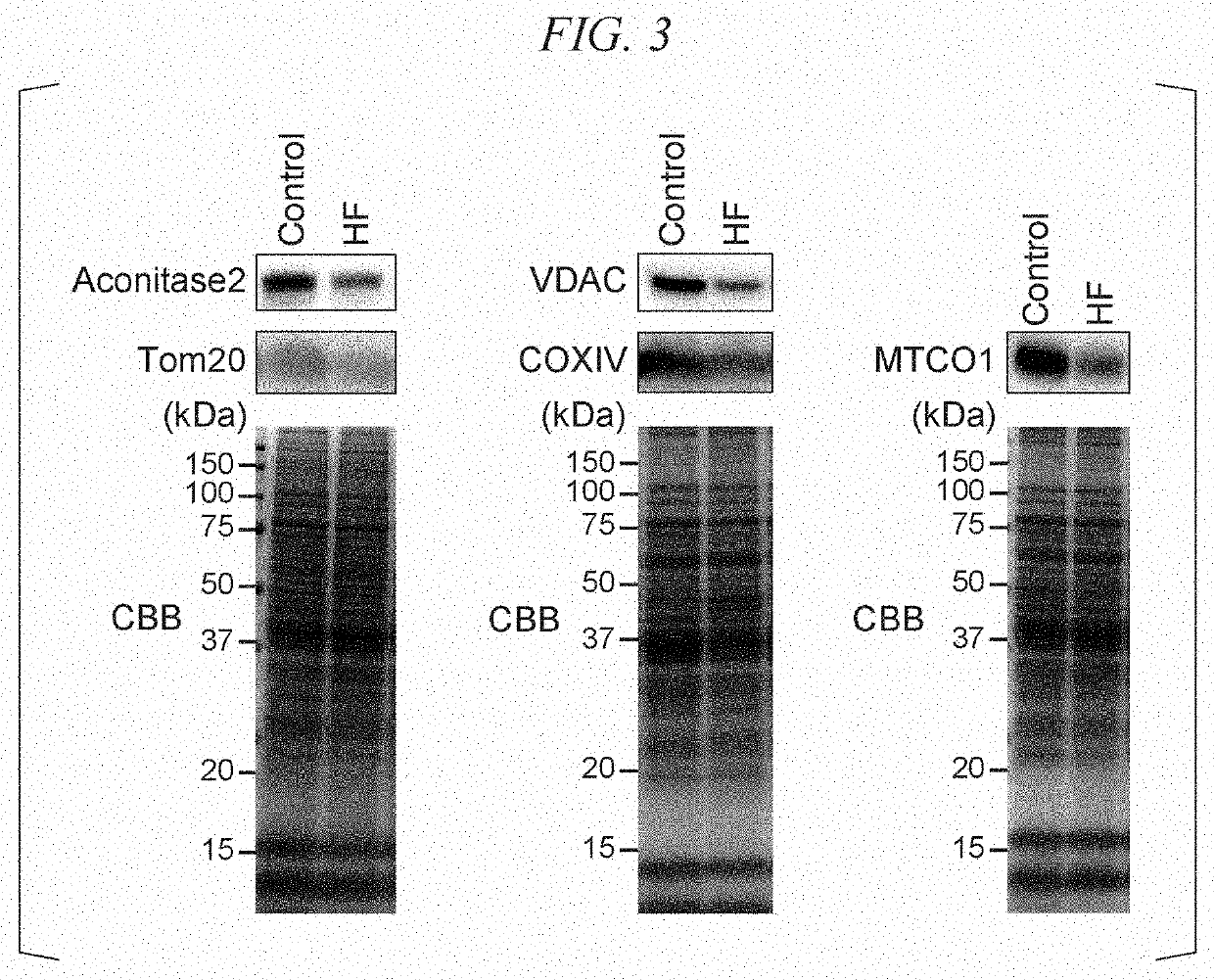
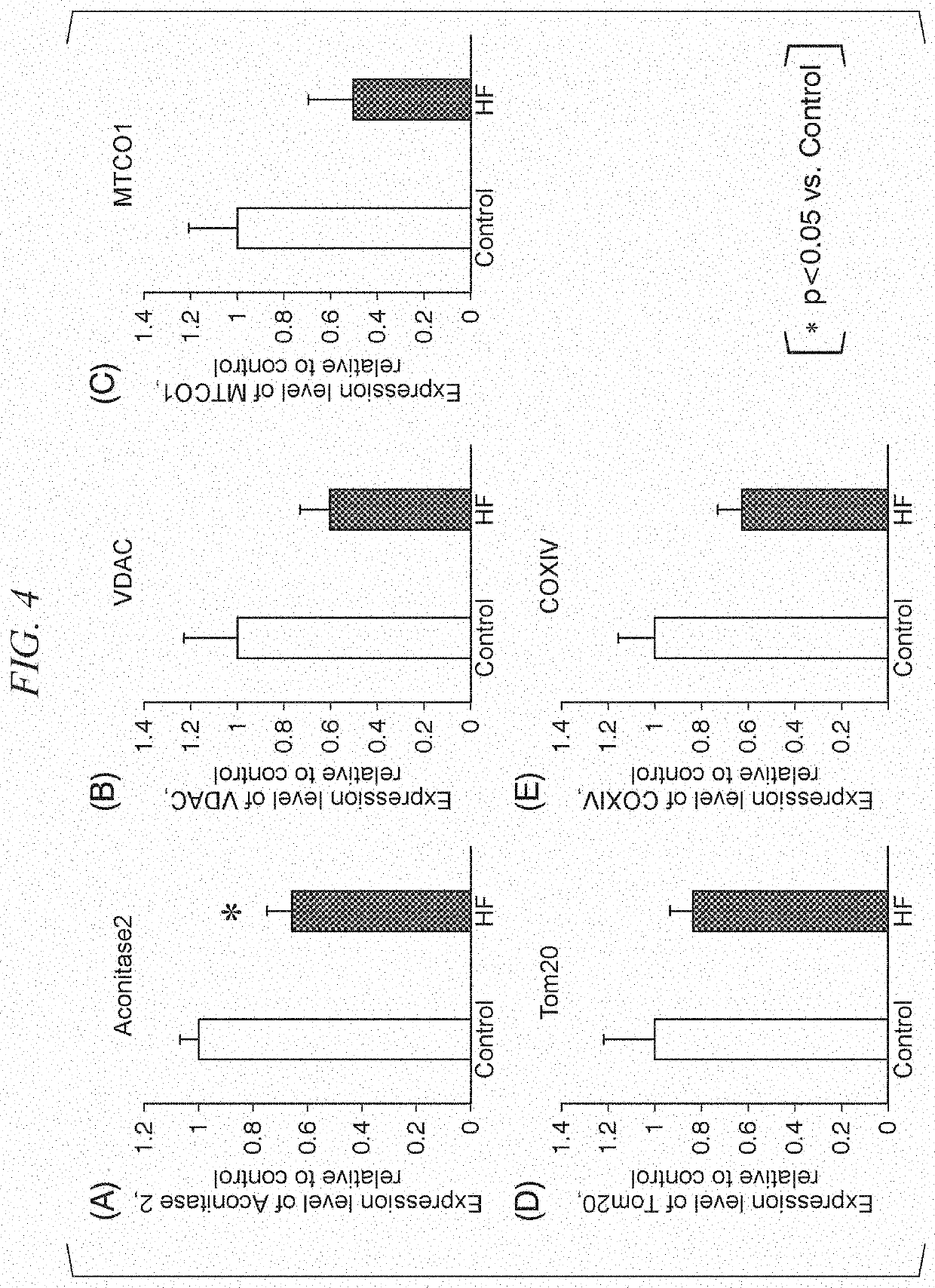
The present invention provides a method of evaluating pathological conditions of heart failure based on a new mechanism of action of heart failure, a method of evaluating a candidate compound for a heart failure treatment drug using the mechanism of action, and a pharmaceutical composition for treating or preventing heart failure using the mechanism of action. That is, the present invention provides a method of evaluating pathological conditions of heart failure including measuring an amount of succinyl-CoA in cardiomyocytes collected from a test animal, a respiratory capacity of mitochondrial complex II in peripheral blood mononuclear cells collected from the test animal or an amount of reactive oxygen species released from peripheral blood mononuclear cells collected from the test animal; and evaluating the onset of heart failure of the test animal or the severity of heart failure based on the obtained measurement values.
Owner:HOKKAIDO UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com