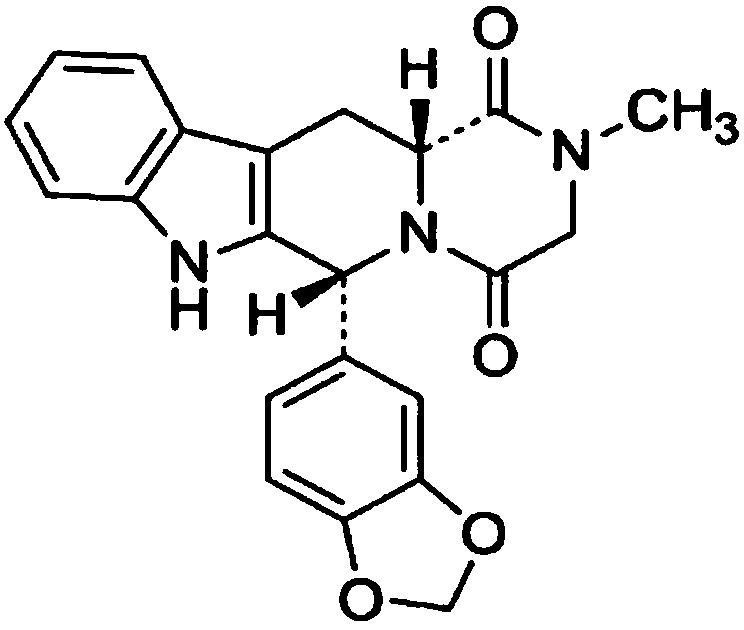
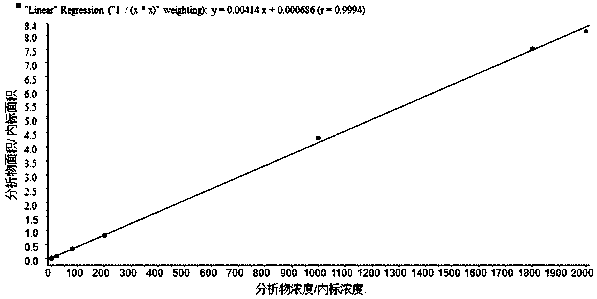Quantitative analysis method for tadalafil in human blood plasma
A technique for quantitative analysis of tadalafil, applied in the field of biological analysis, can solve problems such as low analyte concentration, difficulty in obtaining samples, and large matrix interference, and achieve high repeatability, improved detection sensitivity, and high degree of instrument automation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The preparation of embodiment 1 experimental solution and working solution
[0035] Mobile phase A [referred to as MPA]: Add 1.00 mL of formic acid and about 631 mg of ammonium formate into a glass bottle filled with 1000 mL of ultrapure water, shake and mix to make an aqueous solution containing 0.1% formic acid and 10 mM ammonium formate. Storage conditions: room temperature. Validity period: 2 weeks.
[0036] Mobile phase B [referred to as MPB]: add 1.00mL of formic acid into a glass bottle containing 1000mL of acetonitrile, shake and mix to make a chromatographic grade acetonitrile solution. Storage conditions: room temperature. Validity period: 2 weeks.
[0037] Methanol: water (50:50, v:v) [denoted as R01]: add 500mL methanol and 500mL water into a glass bottle, shake and mix. Storage conditions: room temperature. Validity period: 1 month.
[0038] Methanol: dimethyl sulfoxide (9:1, v:v) [denoted as R02]: Add 18.0mL methanol and 2.00mL dimethyl sulfoxide int...
Embodiment 2
[0058] A kind of LC-MS / MS quantitative analysis method of tadalafil in human plasma, comprises the steps:
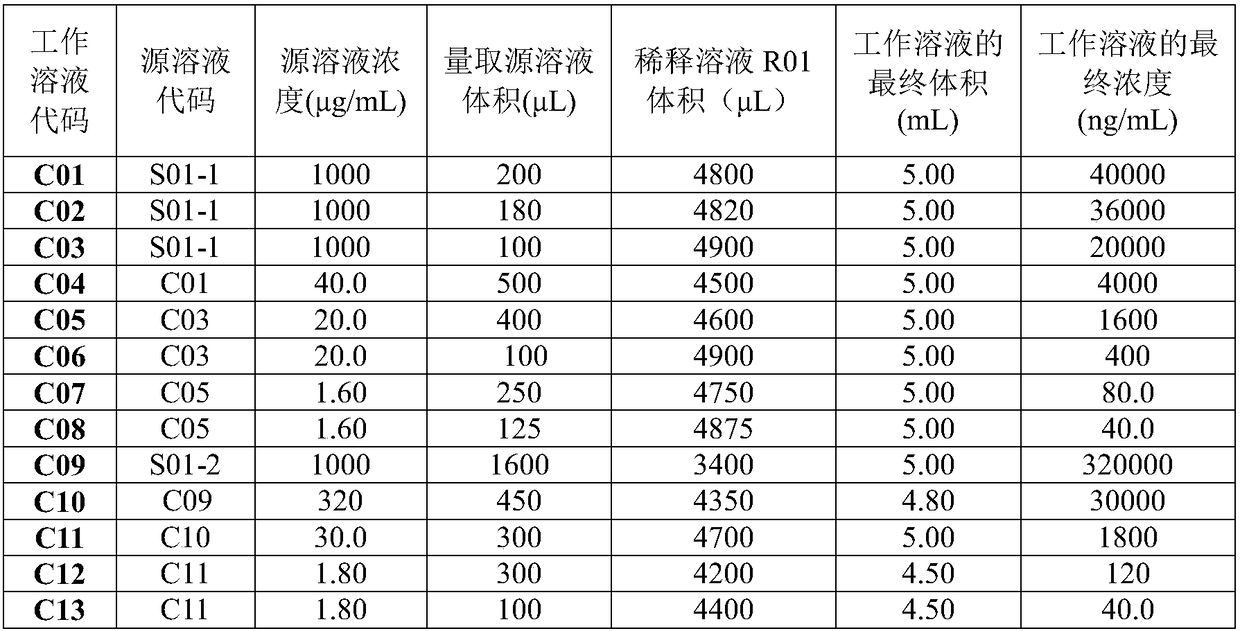
[0059] (1) Preparation of standard working solution: the gradient concentrations of tadalafil working solution were 40.0, 80.0, 400, 1600, 4000, 20000, 36000, 40000ng·mL -1 , the concentration gradient of tadalafil parallel working solution is 40.0, 120, 1800, 30000, 320000ng·mL -1 ;The gradient concentrations of calibration standards are 2.00, 4.00, 20.0, 80.0, 200, 1000, 1800, 2000ng·mL -1 , the concentrations of quality control samples were 2.00, 6.00, 90.0, 1500, 16000ng·mL -1 ;
[0060] Internal standard working solution is 400ng·mL -1 ;Accuracy / stability evaluation solution DIS is 20.0ng·mL -1 , SC1 is 2000ng·mL -1 , SC2 is 20.0ng·mL -1 ;
[0061] The preparation of the above solution only needs to ensure the final concentration, and its preparation method can be based on the preparation method of each solution in Example 1, or can be other methods.
[0062...
Embodiment 3
[0103] This example evaluates the accuracy and precision of the LC-MS / MS method in Example 2 for determining the concentration of tadalafil in human plasma. Take 20.0 μL of the quality control samples of the above four concentrations of QC-2, QC-6, QC-90, and QC-1500, and operate according to the above method, repeat the measurement 3 times for each concentration of 3 samples within 1 day, and Three analysis batches were continuously measured on different days, and the precision was calculated. The results are shown in the following table:
[0104] Analytical batch
[0105] The content LC-MS / MS detection method of tadalafil in the human plasma sample established by the present invention has an extraction recovery rate of more than 75%; the matrix effect is reduced, and the absolute matrix factor reaches between 0.85 and 1.15; the selectivity is enhanced , to reduce the interference on the quantitative analysis of analytes and internal standards; the detection is effi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com