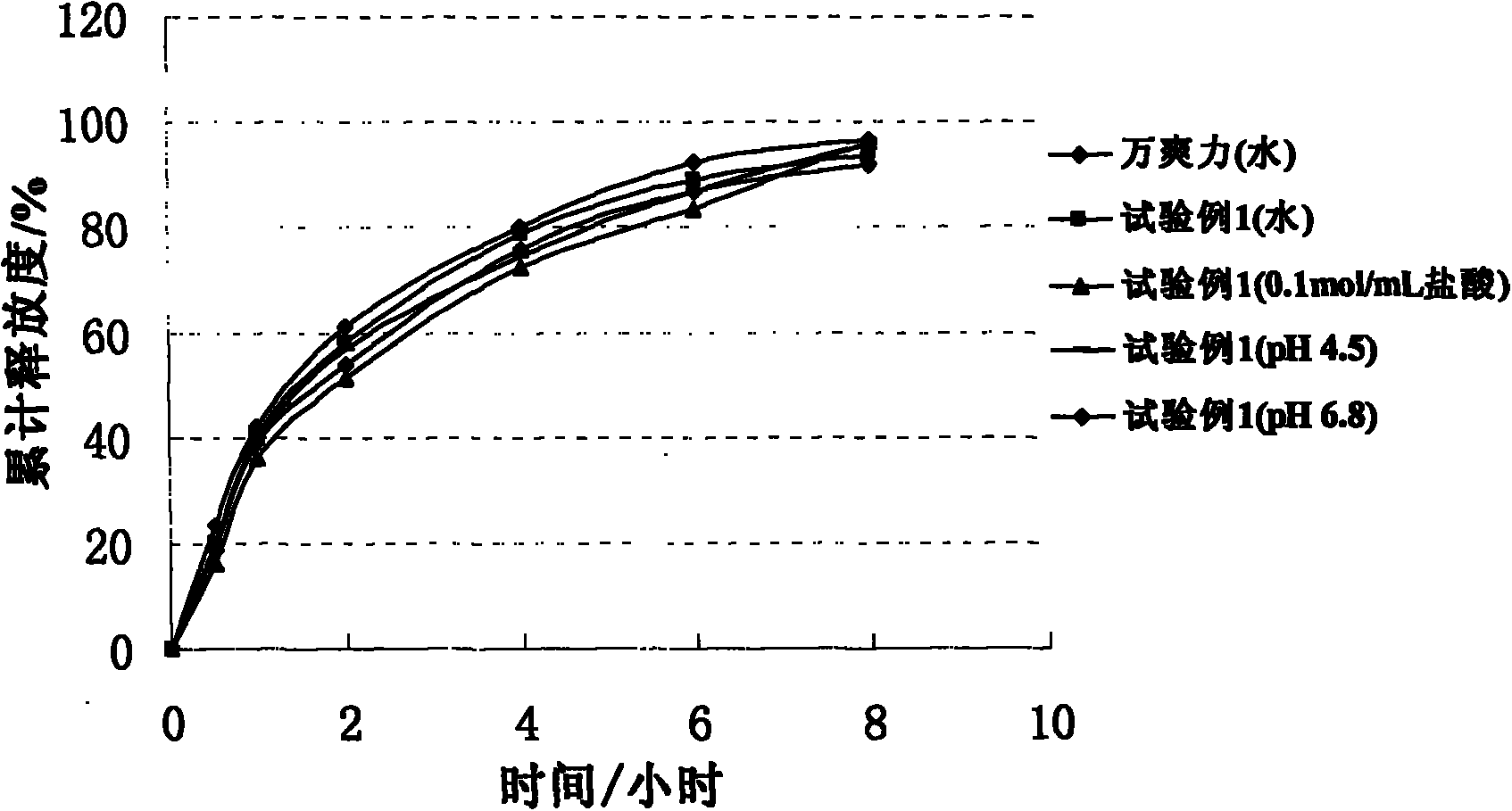
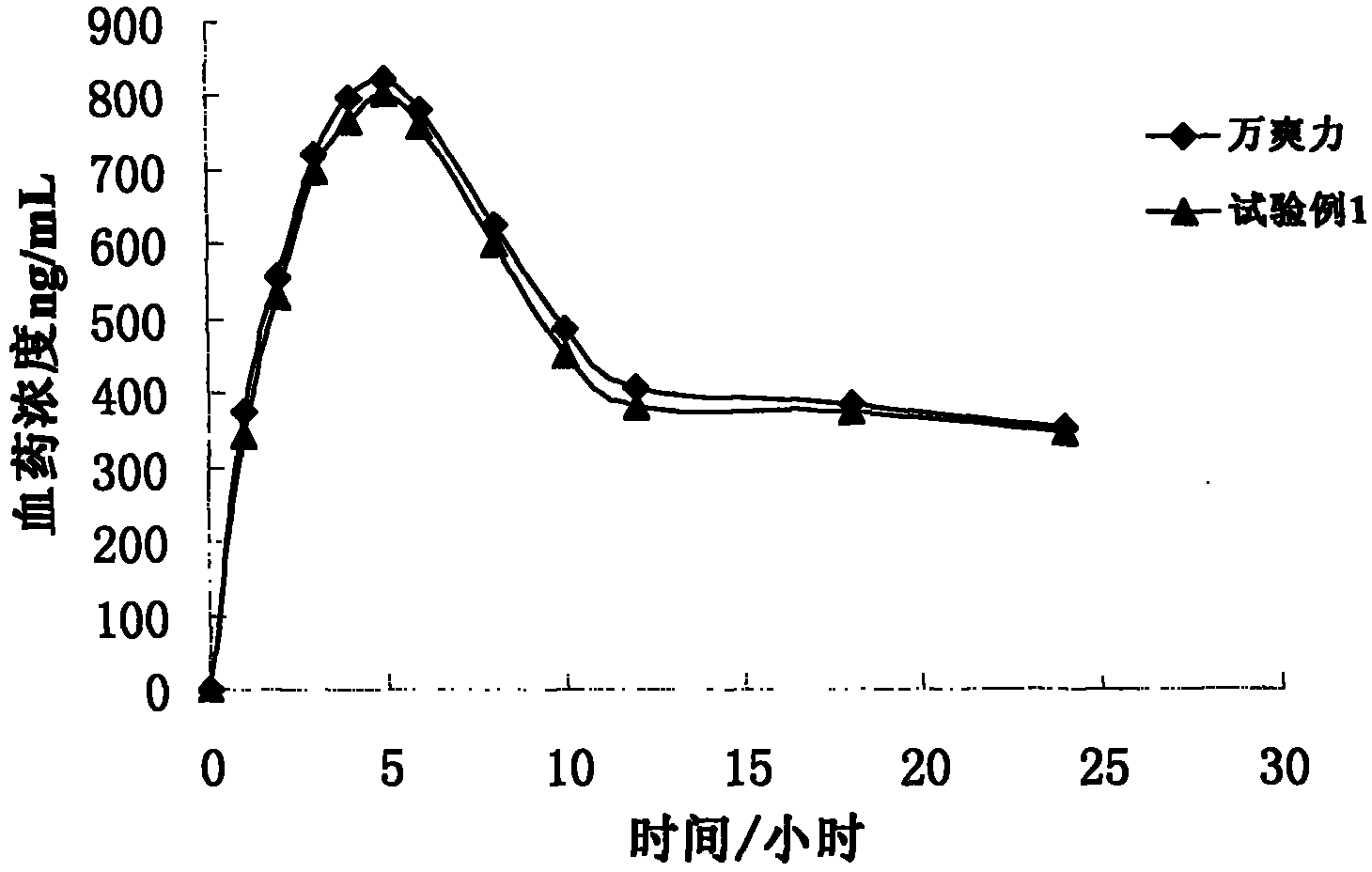
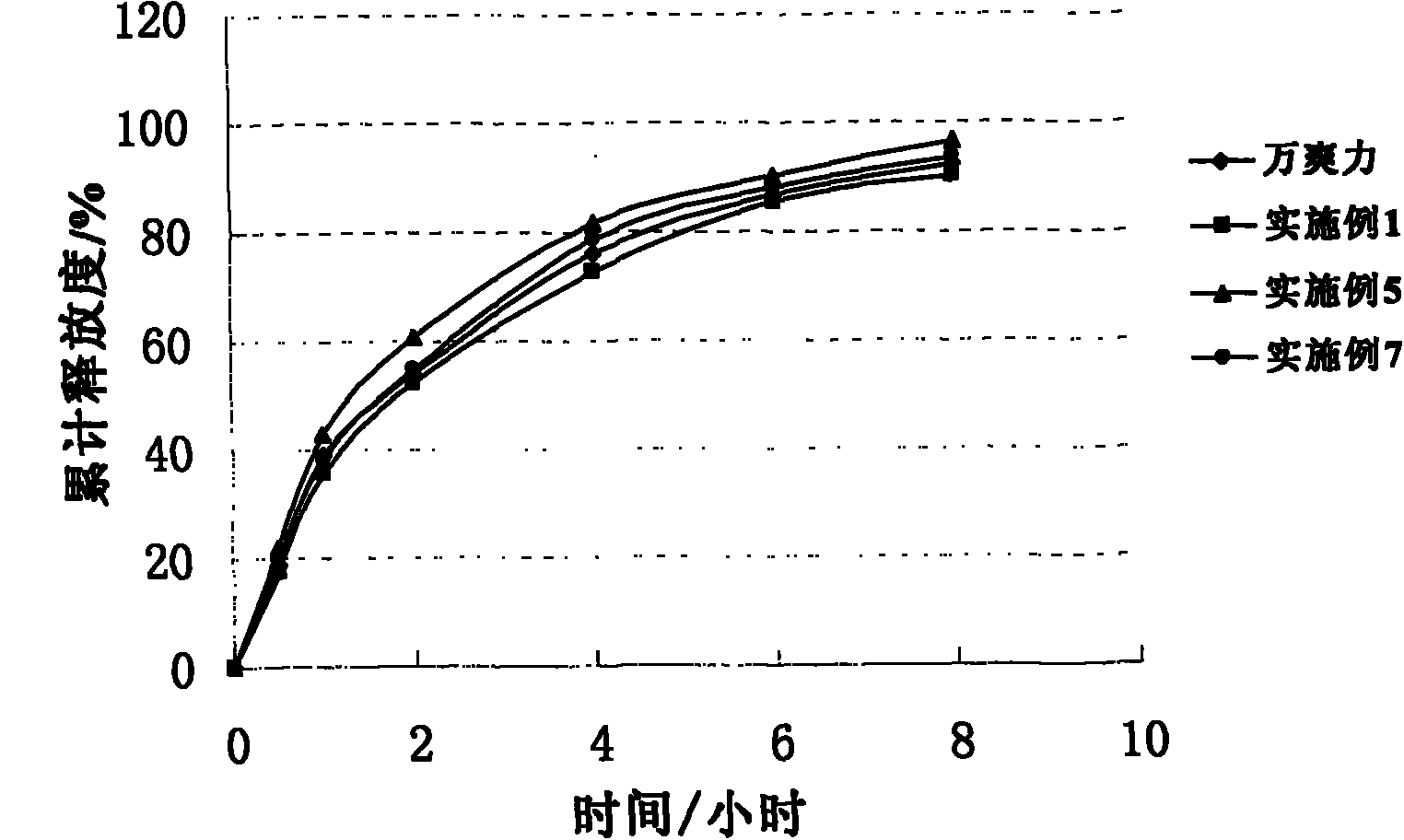
Trimetazidine hydrochloride sustained release tablet taking glyceryl behenate as framework material and preparation method of trimetazidine hydrochloride sustained release tablet
A technology of trimetazidine hydrochloride and glyceryl behenate, which is applied in the field of trimetazidine hydrochloride sustained-release tablets with glyceryl behenate as the skeleton material and its preparation, which can solve the problems affecting the sustained-release effect and application limitations, etc. problem, to achieve the effect of simple process, lower production cost and lower complexity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] A trimetazidine hydrochloride sustained-release tablet, comprising the following components (by 1000 tablets):
[0078]
[0079] The preparation method comprises the following steps:
[0080] (1) Grinding and sieving: trimetazidine hydrochloride is ground into fine powder, get trimetazidine hydrochloride, 888ATO, stearic acid, lactose, microcrystalline cellulose, and calcium hydrogen phosphate are passed through a 60-mesh sieve, and micronized silica gel is passed through a 100-mesh sieve;
[0081] (2) Weighing: Accurately weigh trimetazidine hydrochloride 35.00g, 888ATO120.00g, stearic acid 8.50g, lactose 1.50g, microcrystalline cellulose 25.00g, calcium hydrogen phosphate 9.00g, magnesium stearate 1.00g, spare;
[0082] (3) mixing: the weighed trimetazidine hydrochloride, Mix 888ATO, stearic acid, lactose, microcrystalline cellulose, and calcium hydrogen phosphate in a mixer for 30 minutes;
[0083] (4) Total mixing: add micronized silica gel to the mixed powd...
Embodiment 2
[0086] A trimetazidine hydrochloride sustained-release tablet, comprising the following components (by 1000 tablets):
[0087]
[0088] The preparation method comprises the following steps:
[0089] (1) Grinding and sieving: trimetazidine hydrochloride is ground into fine powder, get trimetazidine hydrochloride, 888ATO, mannitol, lactose, microcrystalline cellulose, calcium hydrogen phosphate pass through a 60-mesh sieve, and talcum powder passes through a 100-mesh sieve;
[0090] (2) Weighing: Accurately weigh trimetazidine hydrochloride 35.00g, 888ATO 140.00g, mannitol 7.00g, lactose 12.00, microcrystalline cellulose 93.00g, calcium hydrogen phosphate 11.00g, talcum powder 2.00g, spare;
[0091] (3) mixing: the weighed trimetazidine hydrochloride, 888ATO, mannitol, lactose, microcrystalline cellulose, and calcium hydrogen phosphate were mixed in a mixer for 30 minutes;
[0092] (4) Total mixing: add talcum powder to the mixed powder and continue mixing for 5 minute...
Embodiment 3
[0095] A trimetazidine hydrochloride sustained-release tablet, comprising the following components (by 1000 tablets):
[0096]
[0097] The preparation method comprises the following steps:
[0098] (1) Grinding and sieving: trimetazidine hydrochloride is ground into fine powder, get trimetazidine hydrochloride, 888 ATOg, stearic acid, mannitol, microcrystalline cellulose, and silicon dioxide are passed through a 60-mesh sieve, and talcum powder is passed through a 100-mesh sieve;
[0099] (2) Weighing: Accurately weigh trimetazidine hydrochloride 35.00g, 888ATO 161.00g, stearic acid 45.00g, mannitol 15.00g, microcrystalline cellulose 132.00g, silicon dioxide 8.00g, talcum powder 4.00g, spare;
[0100] (3) mixing: the weighed trimetazidine hydrochloride, 888ATO, stearic acid, mannitol, microcrystalline cellulose, and silicon dioxide were mixed in a mixer for 30 minutes;
[0101] (4) Total mixing: add talcum powder to the mixed powder and continue mixing for 5 minutes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com