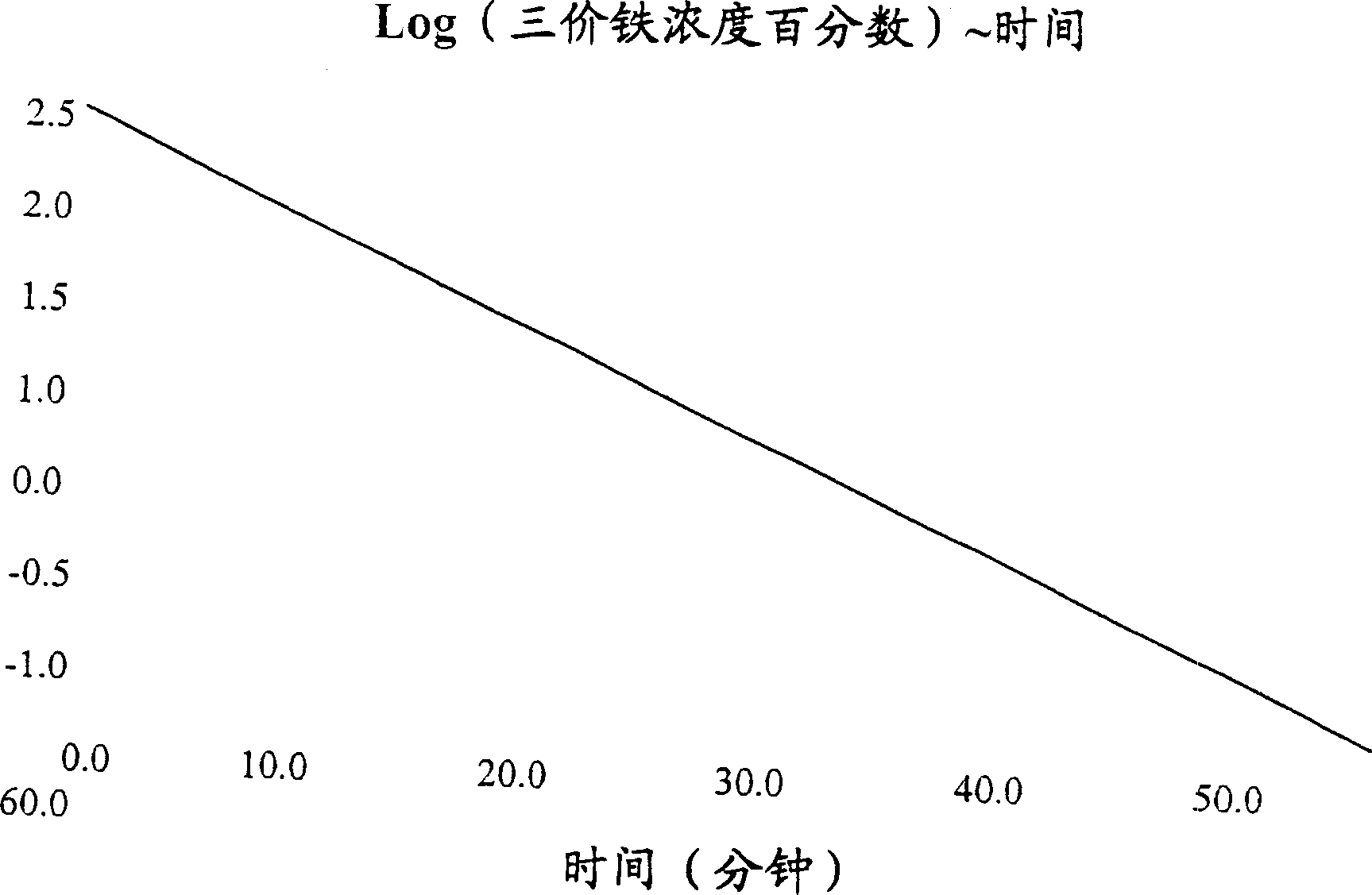
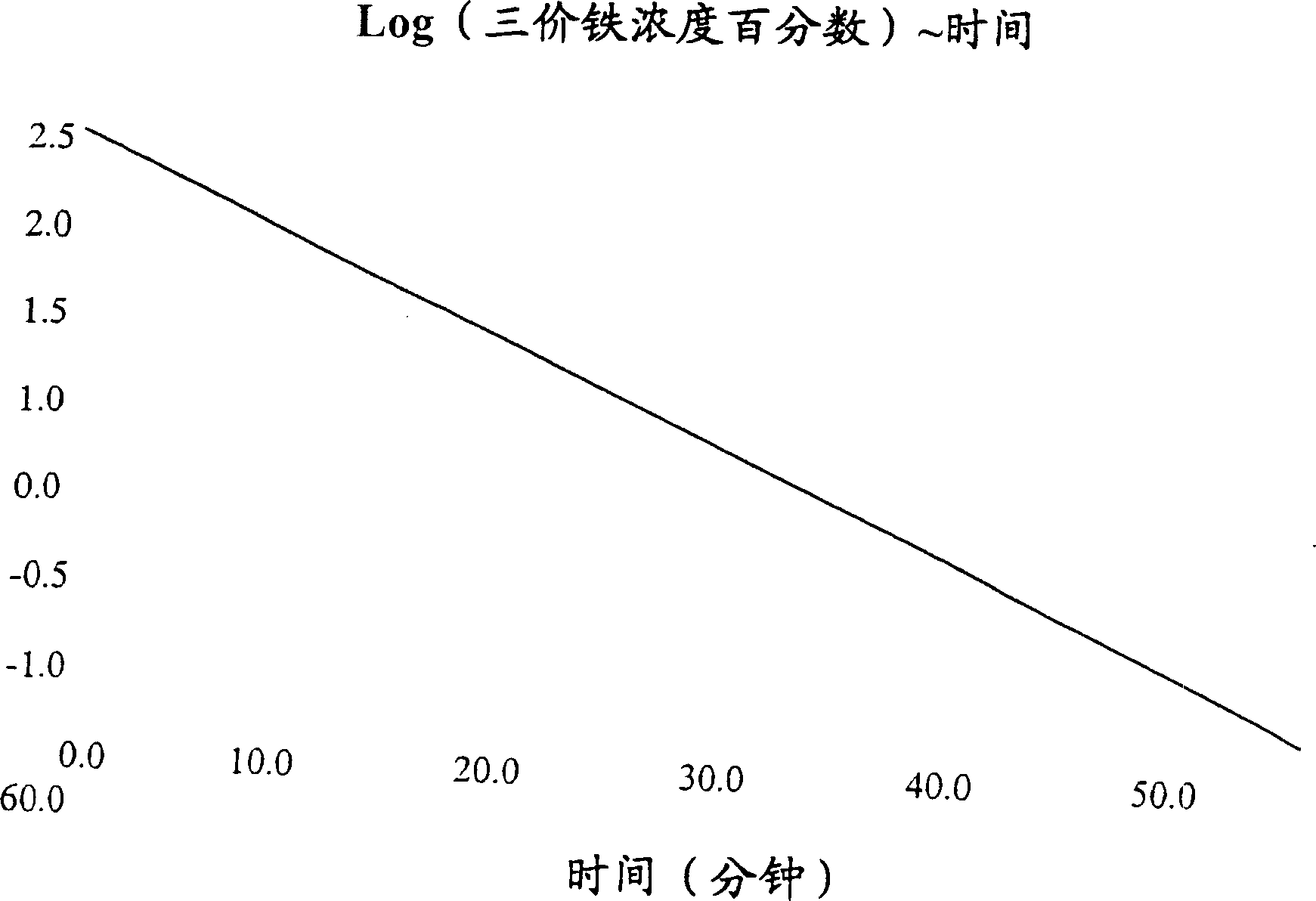
Bioequivalence test for iron-containing formulations
An equivalence and biological technology, applied in biological testing, biological material analysis, medical preparations containing active ingredients, etc., can solve problems such as undefined kinetic parameters such as T
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the application of in vitro test in the control semi-finished product iron sucrose solution:
[0061] At a neutral pH of about 7, a saturated ferric chloride solution was contacted with a 10% w / v sodium carbonate solution. The resulting colloidal ferric hydroxide gel was washed with sufficient purified water to remove any traces of sodium chloride present as a by-product of the reaction.
[0062] A sufficient amount of saturated sucrose solution was added to the colloidal ferric hydroxide gel in a volume equal to that in which the final solution contained about 4.0% w / w elemental iron. The pH of the solution was adjusted to 10.7 with sodium hydroxide, and the solution was mixed at 90°C for 36 hours. QC samples collected during the collection process were used to determine pH, iron content and conduct in vitro bioequivalence tests. If the result is within the allowable limit, add purified water to adjust the volume of the solution so that the final iron c...
Embodiment 2
[0078]Example 2: Application of In Vitro Tests in Controlling Iron Sucrose Solutions Suitable for Injection Use
[0079] An iron sucrose solution suitable for injection was prepared by diluting the semi-finished solution described in Example 1 with water for injection to a final iron concentration of 20 mg / mL. The pH of the resulting solution was adjusted to 10.8 with sodium hydroxide, and then mixed uniformly. The solution was transferred through a stainless steel supply line and filtered through two in-line sterile 0.2 micron filter units into sterile fill bottles. Filters and filling bottles are placed under laminar flow in designated filling chambers with a constant cleanliness class 100.
[0080] All equipment used during filling is identified, sterilized and documented. The corks are washed, siliconized, depyrogenated and sterilized. Glassware was washed with deionized water and finally rinsed with water for injection before depyrogenation.
[0081] The vial filler i...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com