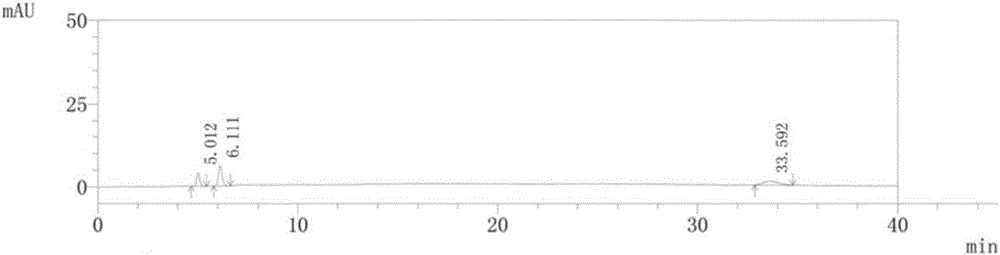
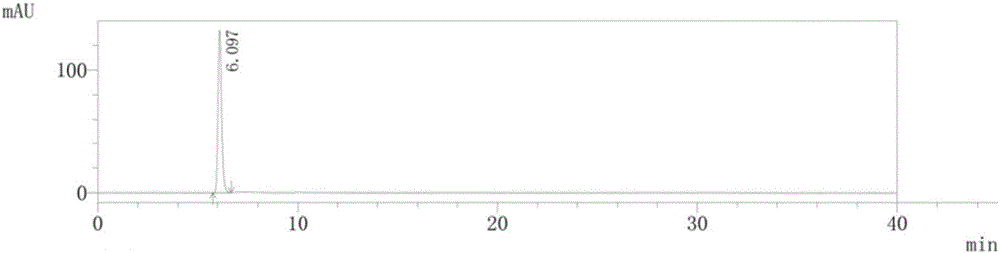
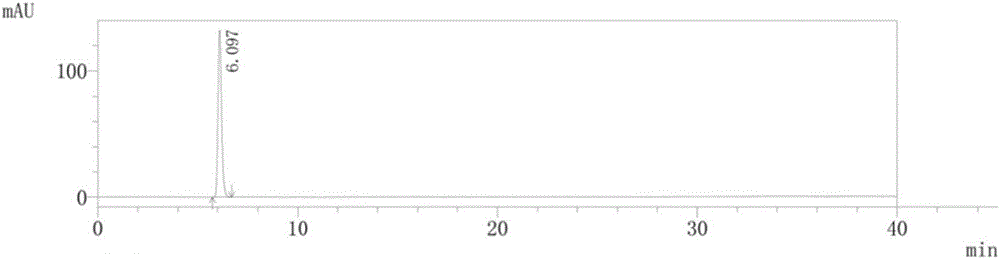
Method for preparing isosorbide mononitrate sustained-release capsules
A technology of isosorbide dinitrate and sustained-release capsules, applied in the directions of capsule delivery, microcapsules, pharmaceutical formulations, etc., can solve the problems of complex production process, inability to effectively prevent the migration of isosorbide mononitrate, poor drug stability, etc., and achieve stable Good properties, good drug safety, and the effect of avoiding easy agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] This example is a specific example of preparing Isosorbide Mononitrate Sustained Release Capsule Product 1.
[0048] Please refer to Table 1 for the formulation composition of Product 1.
[0049] Table 1: The prescription composition of product 1 (4000 capsules)
[0050]
[0051] The specific preparation method of product 1 is as follows.
[0052] Step 1. Fully mix the raw material drug of isosorbide mononitrate and microcrystalline cellulose according to the prescription ratio, and then carry out jet milling treatment to obtain a micronized mixed powder (D90: 10-20 microns), and weigh the above-mentioned micronized powder (wherein the mass ratio of isosorbide mononitrate to microcrystalline cellulose is 2:1) 300.0g is used for powder application; weigh 40.0g HPMC E30 and add 1000g of purified water at room temperature, stir and dissolve until clarification, to obtain Adhesive solution; Take by weighing 400.0g of lactose ball core and place it in the multifunctiona...
Embodiment 2
[0056] This example is a specific example of preparing Isosorbide Mononitrate Sustained Release Capsule Product 2.
[0057] Please refer to Table 2 for the formulation composition of Product 2.
[0058] Table 2: The prescription composition of product 2 (4000 capsules)
[0059]
[0060]
[0061] The specific preparation method of product 2 is as follows.
[0062] Step 1. Fully mix the raw material drug of isosorbide mononitrate and the prescribed amount of microcrystalline cellulose in proportion, then carry out jet milling treatment to obtain a micronized (D90: 10-20 microns) mixed powder, and weigh the above-mentioned micronized powder Powder (the mass ratio of isosorbide mononitrate and microcrystalline cellulose is 10:4) 280.0g, used for powder application; weigh 30.0g of HPMC E30, add 1000g of purified water at room temperature, stir and dissolve until clear , to get the binder solution; take 320.0g of sucrose ball core and place it in the multifunctional pellet c...
Embodiment 3
[0066] This example is a specific example of preparing Isosorbide Mononitrate Sustained Release Capsule Product 3.
[0067] Please refer to Table 3 for the formulation composition of Product 3.
[0068] Table 3: The prescription composition of product 3 (12000 capsules)
[0069]
[0070] The specific preparation method of product 3 is as follows.
[0071] Step 1. Fully mix the raw material drug of isosorbide mononitrate and the prescribed amount of microcrystalline cellulose in proportion, then carry out jet milling treatment to obtain a micronized (D90: 10-20 microns) mixed powder, and weigh the above-mentioned micronized powder Powder (the mass ratio of isosorbide mononitrate and microcrystalline cellulose is 10:3) 780.0g, used for powder application; weigh 60.0g HPMC E30, add 3000g of purified water at room temperature, stir and dissolve until clear, Promptly obtain adhesive solution; Take 800.0g of sucrose ball core and place it in the multi-functional pellet coating ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com