Method for preparing active pharmaceutical ingredient of magnesium aspartate hydrochloride
A technology of magnesium aspartate hydrochloride and aspartic acid, which is applied in the field of preparation of magnesium aspartate hydrochloride raw materials, can solve problems such as unseen research and production reports, and achieve high product yield and simple process , good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
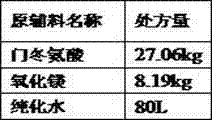
[0026]
[0027]
[0028] Step (a) Add the prescribed amount of purified water into the liquid mixing tank, heat at 50-60°C, add the weighed magnesium oxide under stirring, add aspartic acid in batches while stirring, and continue stirring until the solution is completed clarify;
[0029] Step (b) Suction filter the reaction liquid while it is hot, send it to a multifunctional vacuum dryer, concentrate the filtrate under reduced pressure, remove 30L of water, let the remaining liquid cool down to room temperature naturally, precipitate a large amount of white solid, and centrifuge;
[0030] Step (c) drying at 50-70°C for 4-7 hours to obtain 36.18 kg of solid magnesium aspartate;
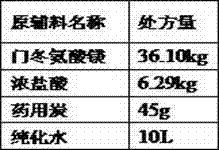
[0031] Step (d) Add 22L of purified water into the ceramic liquid mixing tank, add concentrated hydrochloric acid, and stir well to obtain a hydrochloric acid solution. In the reaction tank, add the prescribed amount of magnesium aspartate and purified water, heat at 40°C, stir for 20 minutes, ...
Embodiment 2
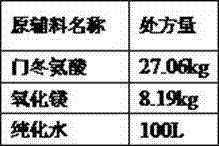
[0036]
[0037]
[0038] Step (a) Add the prescribed amount of purified water into the liquid mixing tank, heat at 50-60°C, add the weighed magnesium oxide under stirring, add aspartic acid in batches while stirring, and continue stirring until the solution is completed clarify;
[0039] Step (b) Suction filter the reaction liquid while it is hot, send it to a multifunctional vacuum dryer, concentrate the filtrate under reduced pressure, remove 30L of water, let the remaining liquid cool down to room temperature naturally, precipitate a large amount of white solid, and centrifuge;
[0040] Step (c) drying at 50-70°C for 4-7 hours to obtain 36.85 kg of solid magnesium aspartate;
[0041] Step (d) Add 22L of purified water into the ceramic liquid mixing tank, add concentrated hydrochloric acid, and stir well to obtain a hydrochloric acid solution. In the reaction tank, add the prescribed amount of magnesium aspartate and purified water, heat at 40°C, stir for 20 minutes, ...
Embodiment 3
[0046]
[0047]
[0048] Step (a) Add the prescribed amount of purified water into the liquid mixing tank, heat at 50-60°C, add the weighed magnesium hydroxide while stirring, add aspartic acid in batches while stirring, and continue stirring until the addition is complete. The solution is clear;
[0049] Step (b) Suction filter the reaction liquid while it is hot, send it to a multifunctional vacuum dryer, concentrate the filtrate under reduced pressure, remove 30L of water, let the remaining liquid cool down to room temperature naturally, precipitate a large amount of white solid, and centrifuge;
[0050] Step (c) drying at 50-70°C for 4-7 hours to obtain 36.78 kg of solid magnesium aspartate;
[0051] Step (d) Add 22L of purified water into the ceramic liquid mixing tank, add concentrated hydrochloric acid, and stir well to obtain a hydrochloric acid solution. In the reaction tank, add the prescribed amount of magnesium aspartate and purified water, heat at 40°C, stir ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com