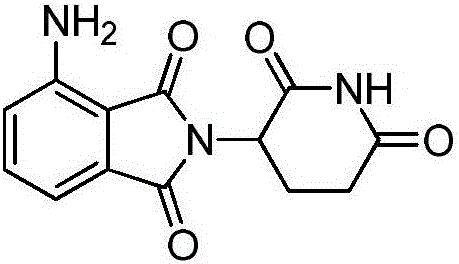
Preparation method for high-purity pomalidomide
A kind of pomalidomide, high-purity technology, applied in the preparation field of high-purity pomalidomide, can solve the problems such as raw material I difficult to react completely, the amount of solvent is large, the yield is low, etc., achieves good inhibitory effect, solvent usage The effect of less and high conversion rate of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
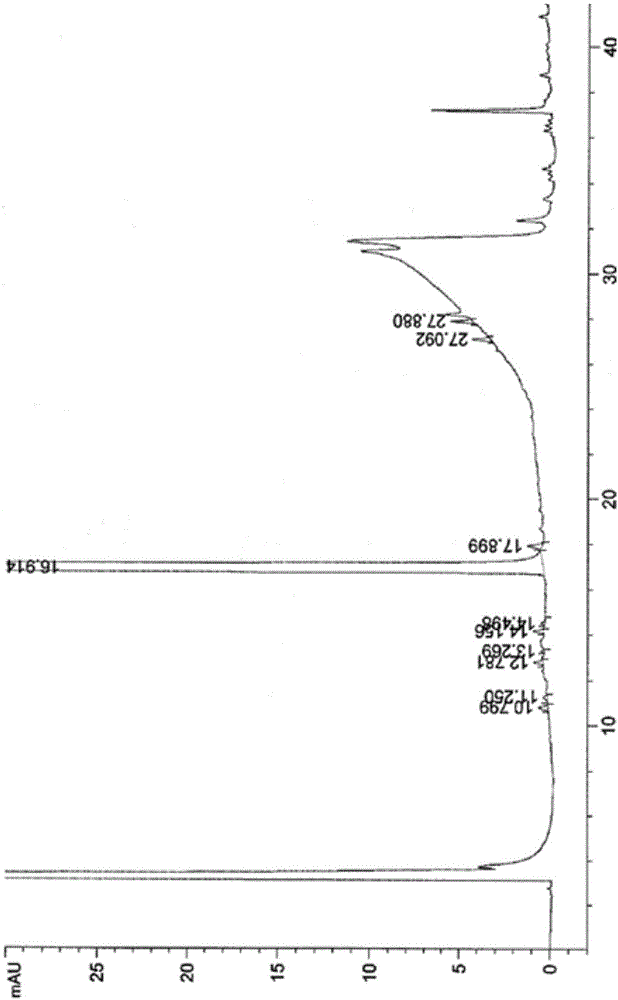
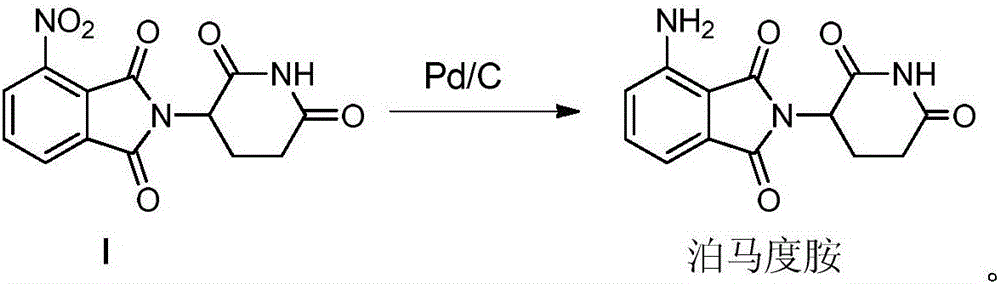
[0054] (1) Add 100g 3-nitro-N-(2,6-dioxo-3-piperidinyl)phthalimide, 10g palladium carbon and 1000ml N-methylpyrrolidone to a 2000ml hydrogenation reactor In the middle, vacuumize, replace with nitrogen for 3 times, then pass in hydrogen, adjust the pressure in the kettle to 0.3Mpa, control the temperature at 45°C, after the reaction is complete, filter with suction, add 30g of medicinal charcoal to the filtrate, heat up to 70°C, and stir for 3 hours. Suction filtration;
[0055] (2) Add the aqueous solution of sodium carbonate (2g sodium carbonate is dissolved in 10ml purified water to make) in step (1) last gained filtrate, stir at room temperature for 0.5 hour, then slowly add dropwise 2000ml purified water, cool to 10 ℃ of crystallization, Suction filtration, the resulting filter cake was added to 500ml of purified water, stirred at room temperature for 0.5 hours, suction filtration, the filter cake was washed with purified water, and the solid was vacuum-dried at 60°C to o...
Embodiment 2
[0057] (1) Add 100g 3-nitro-N-(2,6-dioxo-3-piperidinyl)phthalimide, 10g palladium carbon and 1200ml N-methylpyrrolidone to a 2000ml hydrogenation reactor In the middle, vacuumize, replace with nitrogen for 3 times, then pass in hydrogen, adjust the pressure in the kettle to 0.5Mpa, control the temperature at 50°C, after the reaction is complete, filter with suction, add 30g of medicinal charcoal to the filtrate, heat up to 70°C, and stir for 3 hours. Suction filtration;
[0058] (2) Add an aqueous solution of potassium carbonate (3g of potassium carbonate is dissolved in 12ml of purified water) to the final filtrate of step (1), stir at room temperature for 0.5 hour, then slowly add 2000ml of purified water dropwise, cool to 10°C for crystallization, Suction filtration, the resulting filter cake was added to 500ml of purified water, stirred at room temperature for 0.5 hours, suction filtration, the filter cake was washed with purified water, and the solid was vacuum-dried at 6...
Embodiment 3
[0060] (1) Add 100g 3-nitro-N-(2,6-dioxo-3-piperidinyl)phthalimide, 12g palladium carbon and 1200ml N-methylpyrrolidone to a 2000ml hydrogenation reactor In the process, vacuumize, replace nitrogen for 3 times and then pass in hydrogen, adjust the pressure in the kettle to 0.5Mpa, control the temperature at 50°C, after the reaction is complete, filter with suction, add 30g of medicinal charcoal to the filtrate, heat up to 70°C, and stir for 6 hours. Suction filtration;
[0061] (2) Add an aqueous solution of sodium carbonate (2g sodium carbonate is dissolved in 12ml purified water to make) in step (1) last gained filtrate, stir at room temperature for 0.5 hour, then slowly add dropwise 2000ml purified water, cool to 10 ℃ of crystallization, Suction filtration, the resulting filter cake was added to 500ml of purified water, stirred at room temperature for 0.5 hours, suction filtration, the filter cake was washed with purified water, and the solid was vacuum-dried at 70°C to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com