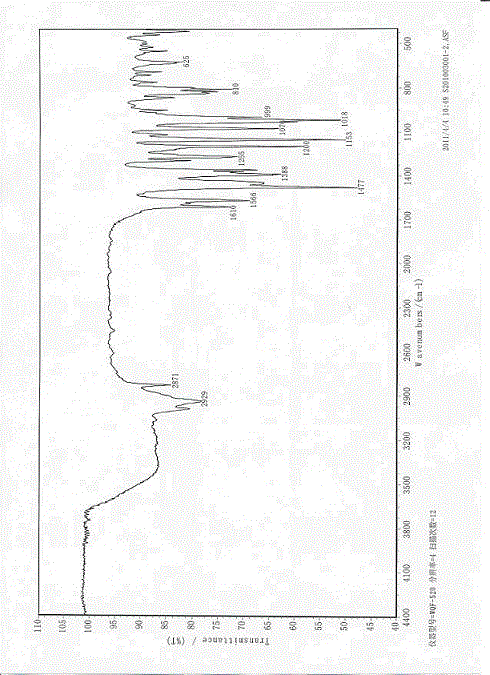
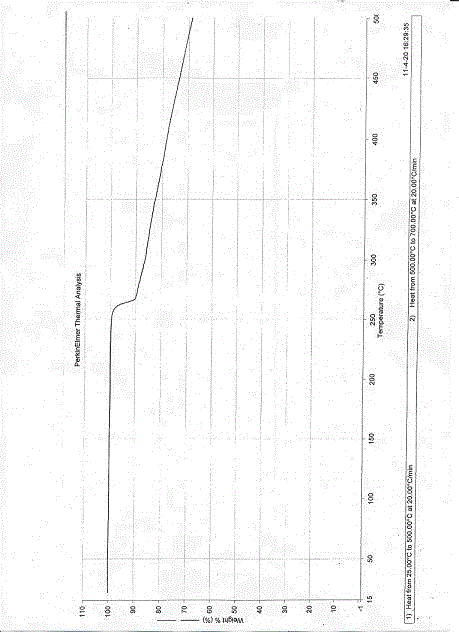
Esomeprazole sodium polymorph and application of esomeprazole sodium polymorph in drugs for injection
A technology for esomeprazole sodium and polymorphic forms, which is applied in the field of medicinal chemistry, can solve the problems of inability to guarantee the consistency of the crystal form of industrial production samples, inability to obtain a high-purity single isomer, poor repeatability in industrial production, and the like, To achieve the effect of easy control of product purity, good quality reproducibility and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0093] Embodiment 1: the preparation of esomeprazole sodium polymorph
[0094] Take 20g of esomeprazole and place it in a reaction flask, add 200mL of acetone, stir thoroughly, dissolve completely after 30min, filter and drain, transfer the filtrate to a 500mL three-neck flask, add hydrogen dropwise at an internal temperature of 20-30°C Sodium methanol solution (2.4g sodium hydroxide / 20mL methanol), after the dropwise addition, continue to stir and react at the same temperature for 6h. Concentrate the reaction solution under reduced pressure at 40°C, stop the concentration when a large amount of solids precipitate out, cool to room temperature (13°C) and crystallize for 4-5 hours, a large amount of white solids precipitate out. Suction filtration, the filter cake was washed with acetone and dried. The solid was dried at 40°C under reduced pressure (above -0.09MPa) to obtain 13.0 g of a white solid, with a yield of 61%. Isomers: not detected, related substances: 0.023%.
...
Embodiment 2
[0096] Embodiment 2: the preparation of esomeprazole sodium polymorph
[0097] Take 200g of esomeprazole and place it in a reaction flask, add 1200mL of acetone, stir well until it is completely dissolved, filter, drain, transfer the filtrate to the reaction flask, add sodium hydroxide methanolic solution dropwise at an internal temperature of 20-30°C ( 24.3g sodium hydroxide / 240mL methanol), after the dropwise addition, continue to stir and react at the same temperature for 6h. The reaction solution was concentrated under reduced pressure at 40°C, and when a large amount of solids were precipitated, the concentration was stopped, cooled to room temperature (5°C), and crystallized for 5 hours, and a large amount of white solids were precipitated. Suction filtration, the filter cake was washed with acetone and dried. The solid was dried at 40°C under reduced pressure (-0.09MPa) to obtain 123.4g of white solid, yield 58%. Isomers: not detected, related substances: 0.035%.
...
Embodiment 3
[0099] Embodiment 3: the preparation of esomeprazole sodium polymorph
[0100] Take 2.03kg of esomeprazole and place it in a reaction bottle, add 20kg of acetone, stir thoroughly, dissolve completely after 30 minutes, filter and drain, transfer the filtrate to a 50L reaction kettle, add hydrogen dropwise at an internal temperature of 20-30°C for oxidation Sodium methanol solution (240g sodium hydroxide / 2L methanol), after the dropwise addition, continue to stir and react at the same temperature for 6h. Concentrate the reaction solution under reduced pressure at 40°C, stop the concentration when there is a large amount of solid, cool to room temperature (25°C) for crystallization, and a large amount of white solid precipitates out. Suction filtration, the filter cake was washed with acetone and dried. The solid was dried at 40°C under reduced pressure (-0.09MPa) to obtain 1.3kg of white solid, yield 60%. Isomers: not detected, related substances: 0.033%.
[0101]
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com