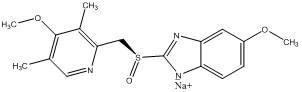
Esomeprazole sodium hemihydrate
A technology of esomeprazole sodium and water compound, applied in the field of medicine, can solve the problems of high total amount of impurities, large number of esomeprazole sodium impurities, low optical purity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Add 60 grams of esomeprazole sodium and 300 milliliters of water into a 500ml reaction bottle equipped with stirring, a thermometer and a condenser, add 0.5 grams of dimethylformamide (DMF) to the above aqueous solution, stir for 30 minutes, and filter , the filtrate was cooled to 12°C and set aside.
[0059] Cool 1150ml of acetonitrile-methyl ethyl ketone = 5:5 mixture to 13°C, add the above standby solution while stirring, keep warm for 18 hours, crystals precipitate, filter, and dry to obtain 53.3 grams of white crystals. Measured 3 times by Karl Fischer method, get the average value, contain 2.40% (weight percent) moisture. Purity 99.9% (HPLC normalization method), optical purity 99.96% ee (chiral HPLC).
[0060] Elemental Analysis Results:
[0061] Measured value (calculated value), C:54.28(54.25),H:5.16(5.09),N:11.21(11.16),
[0062] S: 8.59 (8.52), Na: 6.19 (6.11).
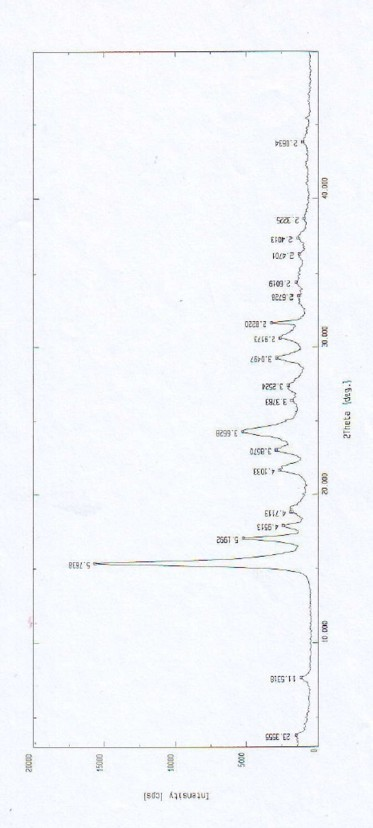
[0063] The X-ray diffraction pattern of the crystal is shown in figure 1 . Instrument model...
Embodiment 2
[0066] Injection containing esomeprazole sodium hemihydrate
[0067] Prescription: Esomeprazole Sodium Hemihydrate (20g as anhydrous substance), 100g mannitol, 140g lactose, 500ml water for injection, made into 1000 sticks.
[0068] Process: Take esomeprazole sodium hemihydrate, mannitol, lactose, and water for injection and stir to dissolve, then add 4g of activated carbon, stir at room temperature for 10 minutes, filter out the activated carbon, and use a microporous membrane to filter bacteria. Pack 1000 tubes, pre-freeze for 3 hours, freeze-dry under reduced pressure for 20 hours, then warm to room temperature, and seal.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com