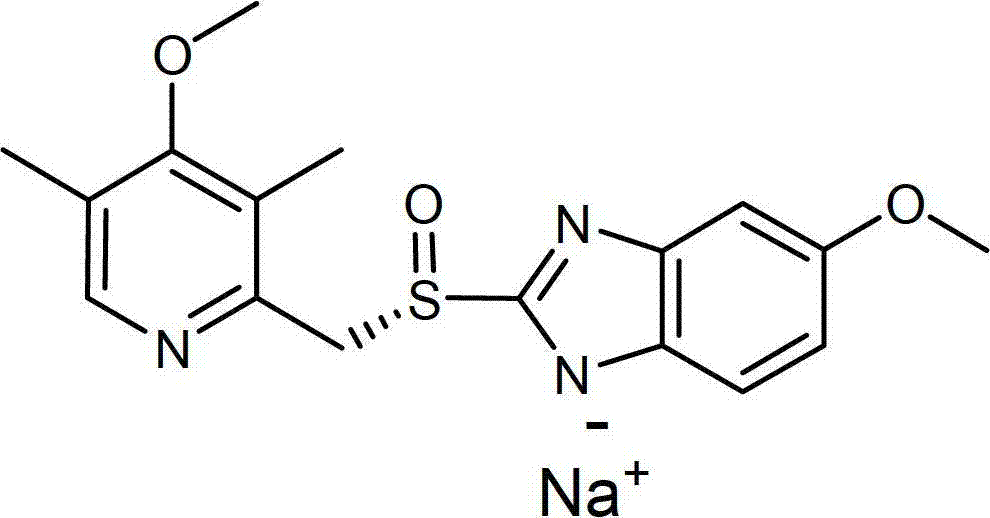
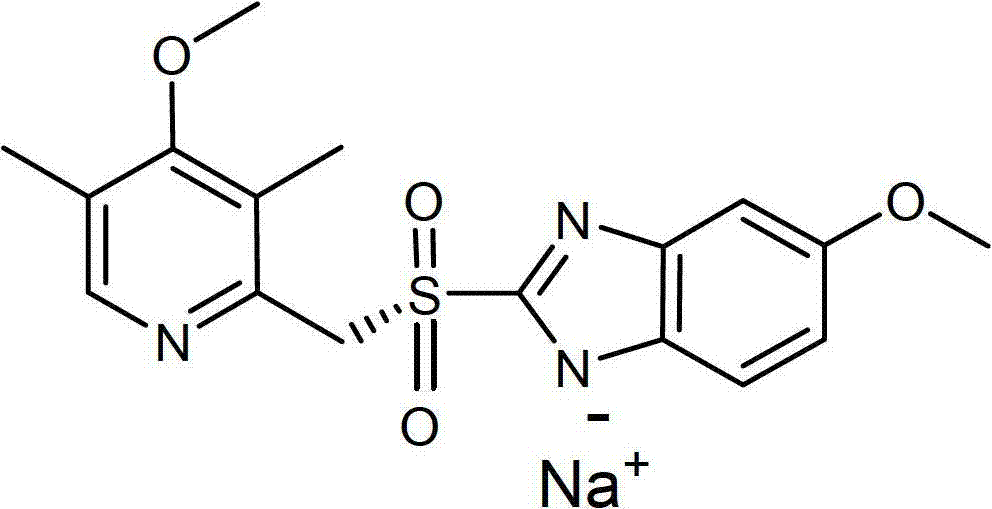
Preparation process of the sodium salt of esomeprazole
A technology of esomeprazole and sodium alkoxide, which is applied in the field of esomeprazole sodium, can solve the problems of unsuitable industrial scale preparation, difficult stirring, and difficult removal of residual solvent due to the degradation of crude substances.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0065] Embodiment 1: use the methanol solution of methyl isobutyl ketone and sodium methylate as solvent to prepare esomeprazole sodium
[0066] 30% sodium methoxide in methanol (4.7 mL, 25.3 mmol) was added dropwise to a solution of esomeprazole (8.5 g, 24.6 mmol; 2.2% sulfone) in methyl isobutyl ketone (60 mL) at room temperature. solution. The resulting slurry was stirred overnight. The solid was collected by filtration, washed with methyl isobutyl ketone (2×10 mL) acetone, and dried under reduced pressure at 50° C. to obtain esomeprazole sodium. Yield: 6.3 g (70%). Sulfone content: 0.33%.
Embodiment 2
[0067] Example 2: Preparation of Emmy using methyl isobutyl ketone / acetone and methanolic sodium methoxide Larazole Sodium
[0068] 30% sodium methoxide in methanol (4.7 mL, 25.3 mmol) was added dropwise to esomeprazole (8.5 g, 24.6 mmol; 2.2% sulfone, 4.7% R-enantiomer) in methylisobutyl solution in ketone (60 mL). The mixture was stirred for 15 minutes. Acetone (60 mL) was added over 30 minutes. The resulting slurry was stirred overnight at room temperature. The solid was collected by filtration, washed with acetone (2 x 10 mL), and dried under reduced pressure at 50 °C to afford esomeprazole sodium. Yield: 5.5 g (61%). Sulfone content: 0.17%. R-enantiomer content: 0.00%.
Embodiment 3
[0069] Example 3: Preparation of Emmy using methyl isobutyl ketone / acetone and methanolic sodium methoxide Larazole Sodium
[0070] 30% sodium methoxide in methanol (6.0 mL, 32.3 mmol) was added dropwise to esomeprazole (10.7 g, 31.0 mmol; 1.1% sulfone, 2.5% R-enantiomer) in methylisobutyl solution in ketone (75 mL). The mixture was stirred for 20 minutes. Acetone (75 mL) was added over 30 minutes. The resulting slurry was stirred overnight at room temperature. The solid was collected by filtration, washed with acetone (2 x 12.5 mL), and dried under reduced pressure at 50 °C to give esomeprazole sodium. Yield: 6.8 g (60%). Sulfone content: 0.06%. R-enantiomer content: 0.00%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com