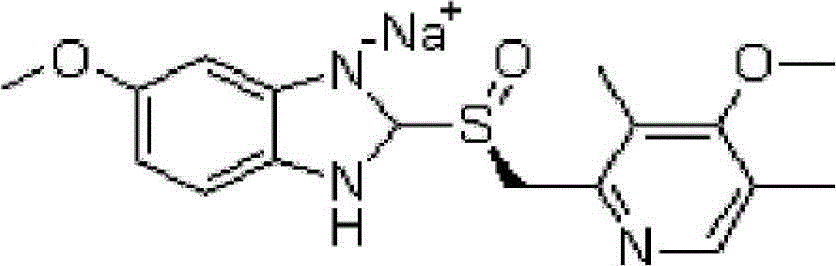
Esomeprazole sodium lyophilized powder injection and preparation method thereof
A technology of esomeprazole sodium and freeze-dried powder injection, which is applied in the field of pharmaceutical preparation, can solve the problems of complex process and equipment system, affecting the physical and chemical stability of esomeprazole sodium liquid medicine, and low feasibility , to achieve the effect of ensuring safety and effectiveness, making the preparation method simple and feasible, and improving feasibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Embodiment 1: Preparation of esomeprazole sodium freeze-dried powder injection
[0035]
[0036]Preparation process: Weigh the prescribed amount of edetate disodium and dissolve it in 990ml of water for injection, adjust the pH of the solution to 12.0 with 20% (g / ml) aqueous sodium hydroxide solution; weigh the prescribed amount of esomeprazole sodium Add it to the above solution, stir at room temperature to dissolve it completely; remove pyrogens by ultrafiltration; take the liquid medicine after ultrafiltration for intermediate inspection; pass the inspection result through a 0.22 μm sterile filter membrane for sterilizing filtration; according to the intermediate As a result of the content determination, adjust the filling volume and fill it; take -50°C as the pre-freezing temperature, and the pre-freezing time is 2 hours; then start sublimation, the sublimation temperature is -5°C, and the sublimation time is 12 hours; then proceed at 35°C Drying, the drying time...
Embodiment 2
[0037] Embodiment 2: Preparation of esomeprazole sodium freeze-dried powder injection
[0038]
[0039]
[0040] Preparation process: Weigh the prescribed amount of edetate disodium and dissolve it in 950ml water for injection, adjust the pH of the solution to 11.5 with 5% (g / ml) sodium hydroxide aqueous solution; weigh the prescribed amount of esomeprazole sodium Add to the above solution, stir at room temperature to dissolve completely, then add 30ml of water for injection, mix evenly; remove pyrogen by ultrafiltration; take the liquid after ultrafiltration for intermediate inspection; pass the test result through a 0.22μm sterilizing filter membrane Carry out sterilizing filtration; adjust the filling volume according to the determination result of the intermediate content and carry out filling; take -40°C as the pre-freezing temperature, and the pre-freezing time is 3 hours; then start sublimation, the sublimation temperature is 0°C, and the sublimation time is 10 ho...
Embodiment 3
[0041] Embodiment 3: Preparation of esomeprazole sodium freeze-dried powder injection
[0042]
[0043] Preparation process: Weigh the prescribed amount of edetate calcium sodium and dissolve it in 970ml of water for injection, adjust the pH of the solution to 11.0 with 10% (g / ml) sodium hydroxide aqueous solution; weigh the prescribed amount of esomeprazole sodium Add it to the above solution, stir at room temperature to dissolve it completely, then add 15ml of water for injection, mix well; remove the pyrogen by ultrafiltration; take the liquid medicine after ultrafiltration for intermediate inspection; pass the test result through a 0.22μm sterilizing filter membrane Carry out sterilizing filtration; adjust the filling amount according to the determination result of the intermediate content and carry out filling; take -45°C as the pre-freezing temperature, and the pre-freezing time is 2.5 hours; then start sublimation, the sublimation temperature is -10°C, and the sublima...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com