Refining method and synthesis method of esomeprazole
A technology for esomeprazole sodium and a purification method is applied in the field of preparation of esomeprazole salts, which can solve the problems of easily causing adverse reactions, multiple impurities and the like, and achieves avoiding adverse reaction events, high purity and high optical purity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
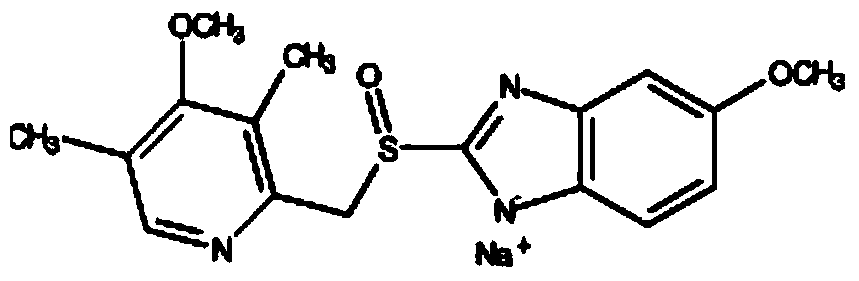
[0063] Example 1: Synthesis of Omeprazole Sulfide
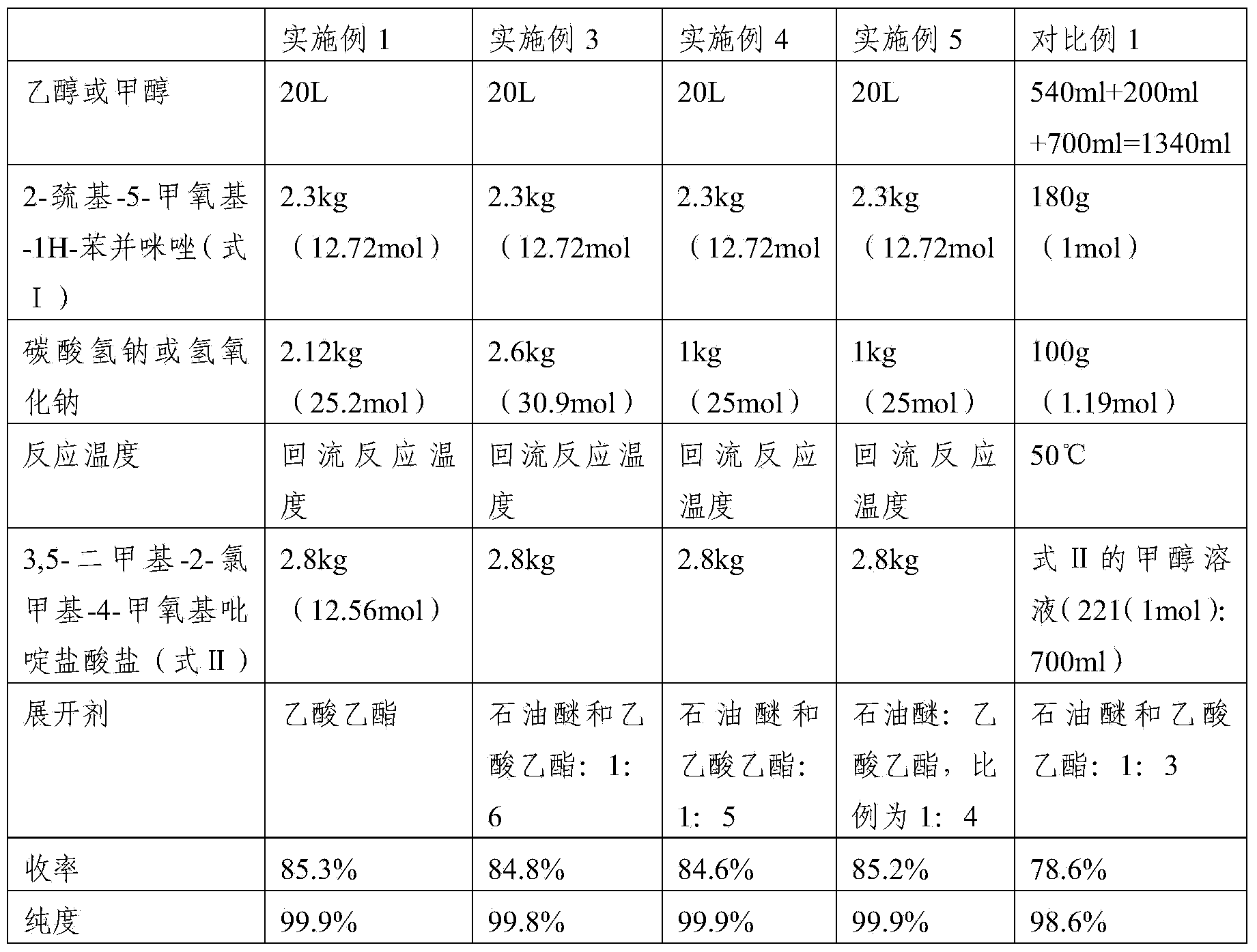
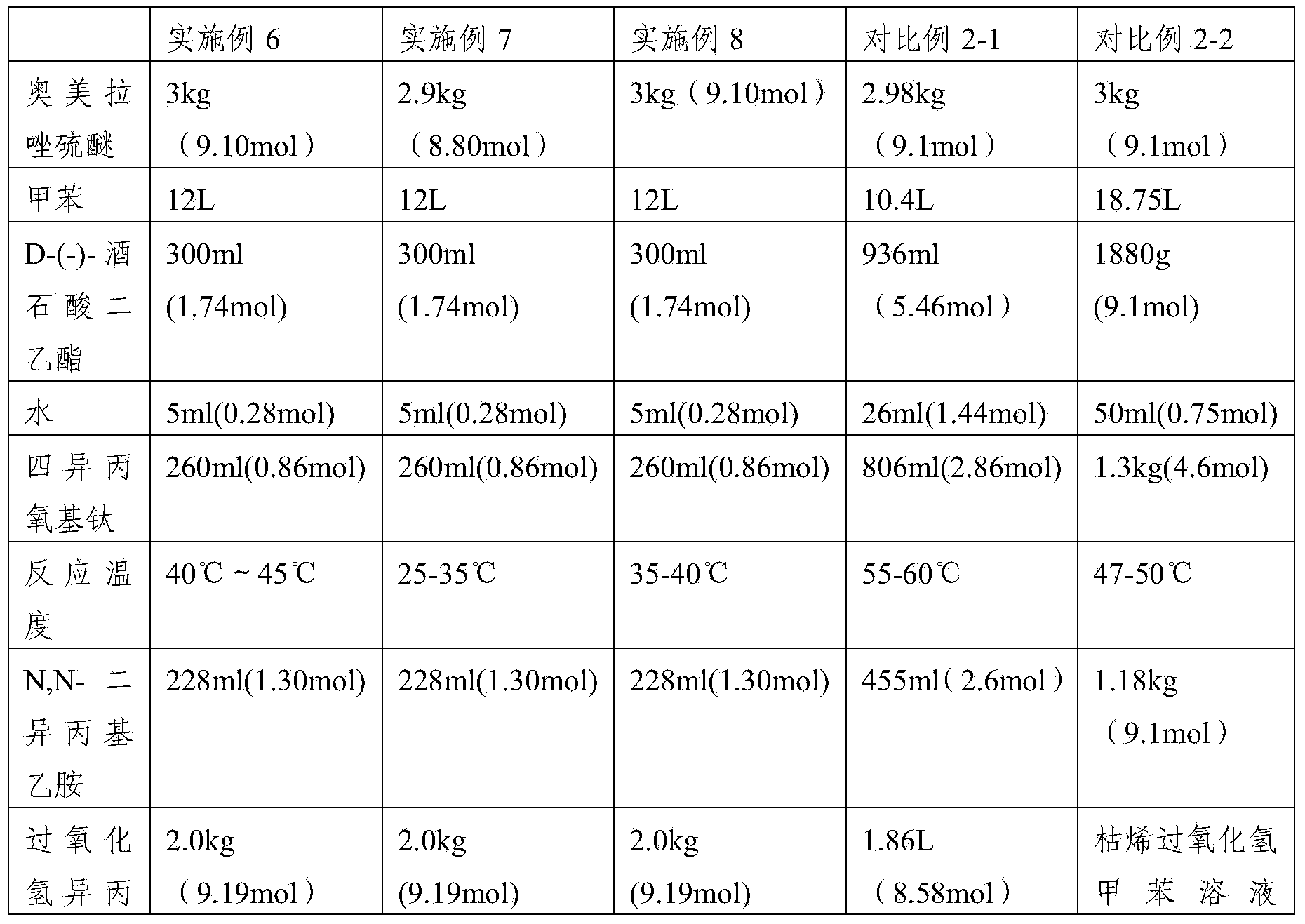
[0064] First add 20L of ethanol (the amount is 8.70 times the volume of the weight of 2-mercapto-5-methoxy-1H-benzimidazole) into the reaction equipment, add 2.12kg (25.2mol) of sodium bicarbonate, after dissolving, add 2- Mercapto-5-methoxy-1H-benzimidazole 2.3kg (12.72mol), after dissolving, add 3,5-dimethyl-2-chloromethyl-4-methoxypyridine hydrochloride 2.8kg (12.56 mol), heat up to reflux reaction (TLC monitoring end point GF254 plate, developing solvent: ethyl acetate) until the raw material point disappears, adjust the pH value to neutrality with acetic acid, filter, and desolvate the filtrate under reduced pressure; add 10 L of dichloromethane to the residue After dissolving, wash with 2L×2 water (that is, use 2L water for 2 times), dry with anhydrous sodium sulfate, filter, and desolvate the filtrate under reduced pressure, add 8L xylene to stir and dissolve the material, add 100g activated carbon, stir for about 15mi...
Embodiment 2
[0065] Example 2: Synthesis of Omeprazole Sulfide
[0066] First, add 20L of methanol (the amount is 8.70 times the volume of the weight of 2-mercapto-5-methoxy-1H-benzimidazole) into the reaction equipment, add 1kg (25mol) of sodium hydroxide, and after dissolving, add 2-mercapto- 5-Methoxy-1H-benzimidazole 2.3kg (12.72mol), add 3,5-dimethyl-2-chloromethyl-4-methoxypyridine hydrochloride 2.8kg (12.56mol) after dissolution , heat up to the reflux reaction (TLC monitoring end point GF254 plate, developing solvent: ethyl acetate) until the raw material point disappears, adjust the pH value to neutrality with acetic acid, filter, and remove the filtrate under reduced pressure; after adding 10L of dichloromethane to dissolve the residue , washed with 2L × 2 water, dried with anhydrous sodium sulfate; filtered, the filtrate was desolvated under reduced pressure, and 8L of xylene was added to dissolve the material, then 100 g of activated carbon was added, stirred for about 15 min, ...
Embodiment 3
[0067] Example 3: Synthesis of Omeprazole Sulfide
[0068] First, add 20 L of methanol (the amount is 8.70 times the volume of the weight of 2-mercapto-5-methoxy-1H-benzimidazole) into the reaction equipment, add 2.6 kg (30.9 mol) of sodium bicarbonate, and after dissolving, add 2- Mercapto-5-methoxy-1H-benzimidazole 2.3kg (12.72mol), after dissolving, add 3,5-dimethyl-2-chloromethyl-4-methoxypyridine hydrochloride 2.8kg (12.56 mol), be warming up to reflux reaction (TLC monitoring GF254 plate, developing agent: petroleum ether and ethyl acetate, the volume ratio of the two is 1:6) to react to the disappearance of the raw material point, adjust the pH value to neutrality with acetic acid, filter, filtrate Precipitate under reduced pressure; add 10L of dichloromethane to dissolve the residue, wash with 2L×2 water, and dry with anhydrous sodium sulfate; filter, remove the filtrate under reduced pressure, add 8L of xylene to stir and dissolve the material, add 100g of activated c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com