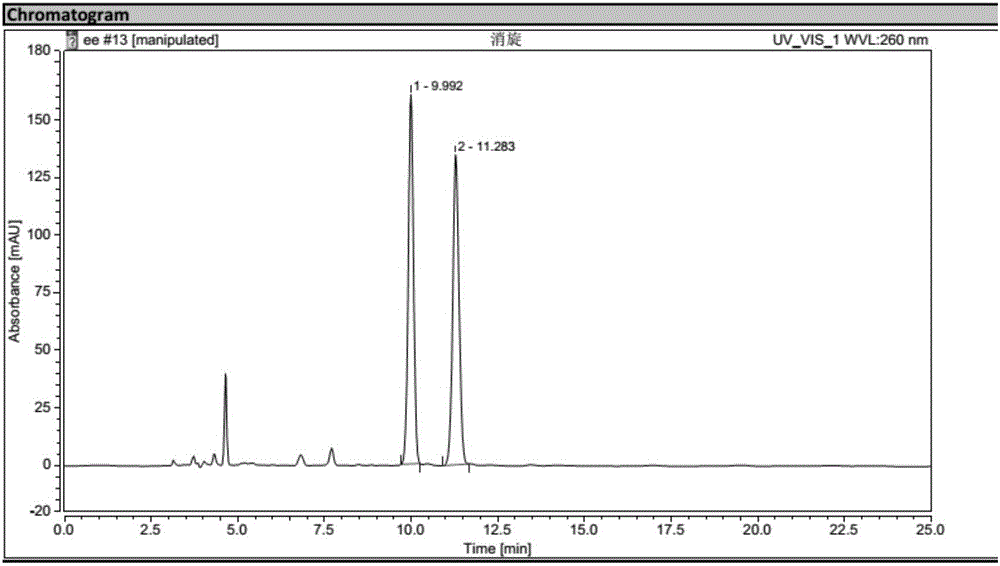
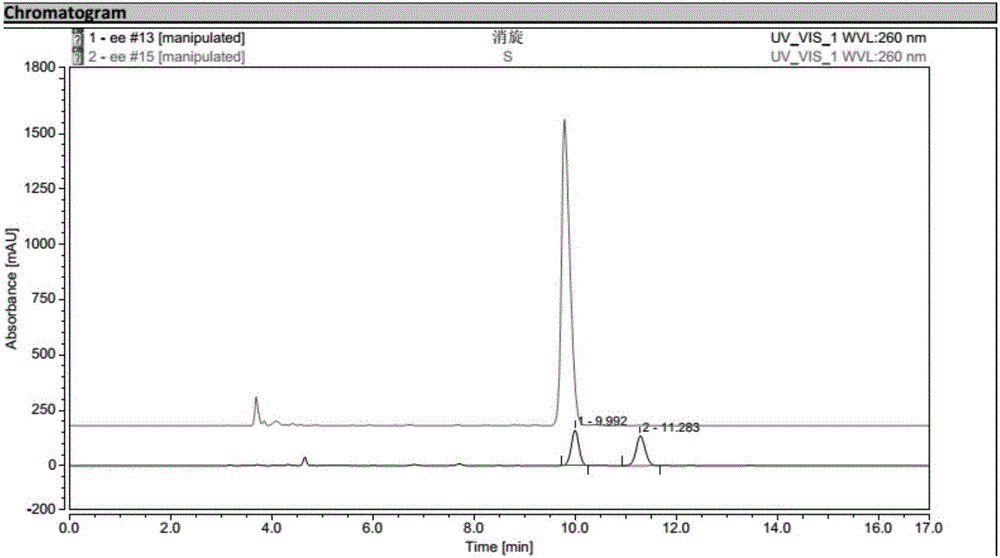
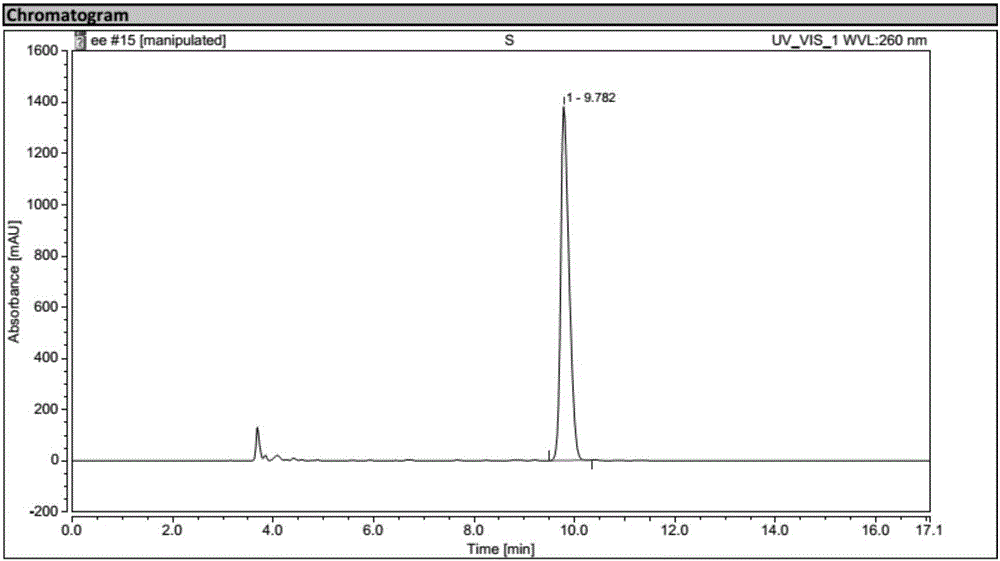
Biological preparation method for (1R, 2S)-2-(3,4-difluorophenyl) cyclopropylamine D-mandelate (I)
An amino acid and carbonyl technology, applied in the field of medicine, can solve the problems of harsh process operating conditions, severe exotherm, difficult product ee value, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0100] The invention provides a preparation method of a compound of formula V, comprising the steps of:
[0101] (a) In a liquid reaction system, the compound of formula VI is used as a substrate, in the presence of a coenzyme, under the catalysis of carbonyl reductase, to perform an asymmetric reduction reaction, thereby forming the compound of formula V;
[0102]
[0103] with
[0104] (b) optionally isolating the compound of formula V from the reaction system after the reaction in the previous step.
[0105] In the present invention, the above reaction can be coupled or not coupled with the coenzyme regeneration system.
[0106] Preferably, the above reactions are coupled with a coenzyme regeneration system in the same system, thereby further improving production efficiency, reducing production costs and increasing tolerance to substrates.
[0107] reaction system
[0108] The present invention also provides a reaction system used in the biological preparation method of...
Embodiment 1
[0166] Embodiment 1, the construction of carbonyl reductase engineering bacteria
[0167]The QNR target gene and alcohol dehydrogenase gene were entrusted to a commercial company to carry out the whole gene synthesis, cloned into the pET28a(+) vector, transformed into Escherichia coli DH5α competent cells, cultured on the plate, picked a single colony of positive transformants and extracted the plasmid After the sequencing is confirmed, the recombinant plasmid is extracted, introduced into the BL21 (DE3) strain, cultured in LB, and the genetically engineered bacteria capable of inducing the expression of the recombinant carbonyl reductase and alcohol dehydrogenase are obtained.
Embodiment 2
[0168] Embodiment 2, the preparation of recombinant carbonyl reductase, alcohol dehydrogenase
[0169] Inoculate the genetically engineered bacterium stored in glycerol in the previous step into the LB liquid medium containing kanamycin, cultivate for 13 hours at 37°C and 220rpm to obtain the seed medium, and inoculate the seed culture medium in a proportion of 1.5%. On the liquid medium containing 50ug / ml kanamycin resistance, then cultivate to OD at 37℃, 220rmp 600 If the value is >2.0, add lactose with a final concentration of 1.0%, cool down to 25°C, continue to cultivate for 3 hours, add lactose with a final concentration of 0.5%, and cultivate for 20 hours, put it in a tank, and centrifuge to obtain bacteria, which is ready for biotransformation.
[0170] The fermentation formula is as follows:
[0171] raw material Mass content (%) Yeast extract 2.4 soy peptone 1.2 Sodium chloride 0.3 glycerin 0.5 Dipotassium phosphate 0.2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com