Preparation method of dimethylmorphinan phosphate used as cough medicine
A technology of dimethylorphantyl phosphate and dichloromethane, which is applied in the direction of organic chemistry, can solve the problems of low chiral resolution yield, high production cost, and many process steps, and achieve the reduction of chiral resolution process and production The effect of short cycle and simple process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
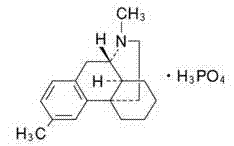
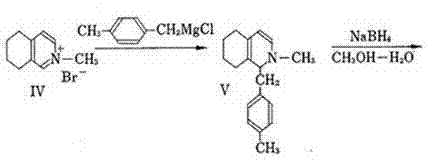
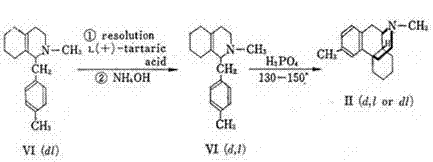
[0029]The preparation method of dimetyl phosphate of the present invention, the method is: (S)-1-(4-methylbenzyl)-1,2,3,4,5,6,7,8-eight De-L-mandelic acid of hydroisoquinoline-L-mandelic acid to obtain (S)-l-(4-methylbenzyl)-1,2,3,4,5,6,7,8-octahydro Isoquinoline, using (S)-l-(4-methylbenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline chiral substance as raw material for methylation , and then undergo a ring-forming reaction with phosphoric acid under heating and reduced pressure to obtain (9S, 13S, 14S)-3,17-dimethylmorphinan monophosphate with three chiral centers, that is, dimethylmorphanyl phosphate , and then go through desalination, rectification, crystallization, salt formation, drying and packaging, and finally obtain the refined product of dimethylorphane phosphate.
[0030] The (S)-l-(4-methylbenzyl)-1,2,3,4,5,6,7,8-octahydroisoquinoline-L-mandelate, the chirality used The protective reagent can also be one of L-tartaric acid, L-(+)-dimethyl tartrate, L-(+)-ethyl ma...
Embodiment 1
[0041] Add 100g of L-D-3 mandelate, 400ml of water and 400ml of dichloromethane into a 1000ml three-necked flask, stir for 0.5h, then add 150g of 10% aqueous sodium bicarbonate solution dropwise, continue stirring for 1h, separate layers and concentrate. Then add 400ml of ethanol, 25g of paraformaldehyde and 8g of 5% palladium carbon, and feed hydrogen to maintain a certain pressure. After reacting for 10 hours, filter and concentrate, then add 200g of phosphoric acid, heat up to 120°C and evaporate water under reduced pressure, keep warm for 50 hours, adjust pH to 8 with ammonia water, extract with 400ml of dichloromethane, and concentrate. In high vacuum, rectification under the condition of 170 ℃. Add 5g of phosphoric acid to finally obtain 25g of white crystals with a content of 99.2%.
Embodiment 2
[0043] Add 100g of L-D-3 mandelate, 400ml of water and 400ml of dichloromethane into a 1000ml three-necked flask, stir for 0.5h, then add 250g of 10% aqueous sodium carbonate solution dropwise, continue stirring for 1h, separate layers and concentrate. Then add 400ml of ethanol, 30g of paraformaldehyde and 7g of 5% palladium carbon, and feed hydrogen to maintain the pressure. After reacting for 22 hours, filter, concentrate, add 300g of phosphoric acid, heat up to 110°C and evaporate water under reduced pressure, keep warm for 40-80h, adjust pH to 9 with ammonia water, extract with 400ml of dichloromethane, and concentrate. In high vacuum, rectification under the condition of 170 ℃. Add 10g of phosphoric acid to finally obtain 52g of white crystals with a content of 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com