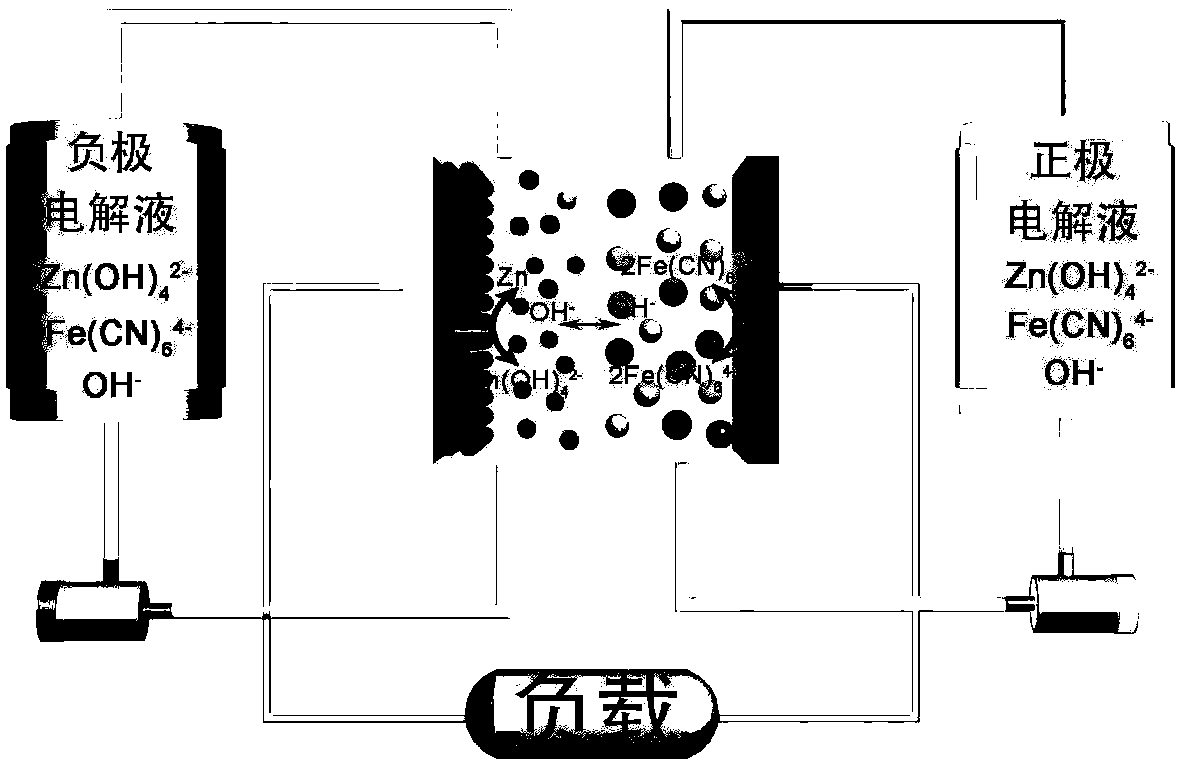
Alkaline zinc-iron electrochemical flow cell
A liquid flow battery, zinc-iron technology, applied in the direction of alkaline electrolyte, fuel cell, battery electrode, etc., can solve the problems of increasing battery maintenance cost, battery voltage efficiency drop, battery energy efficiency attenuation, etc., to reduce electrolyte migration problems, system maintenance cost reduction, effect of high open circuit voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
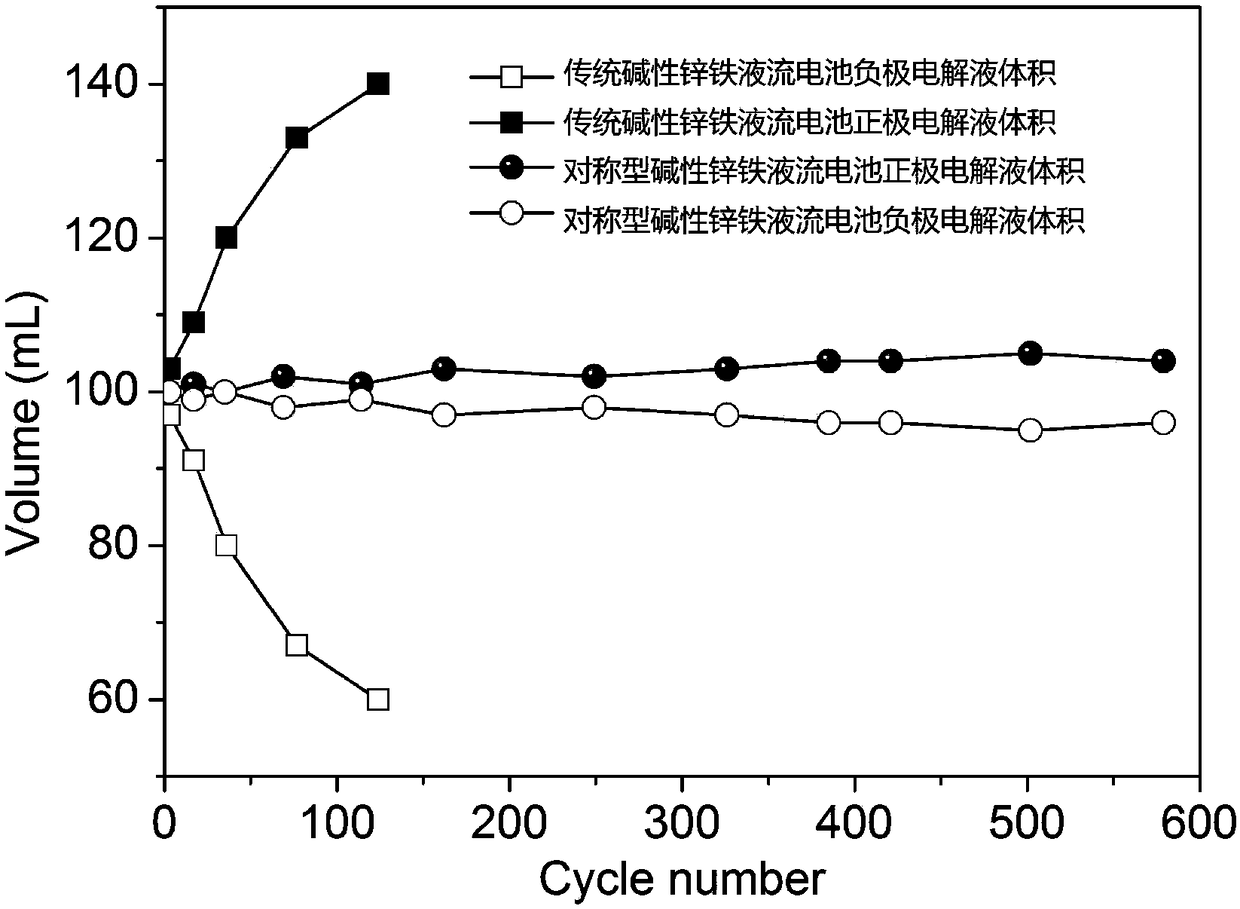
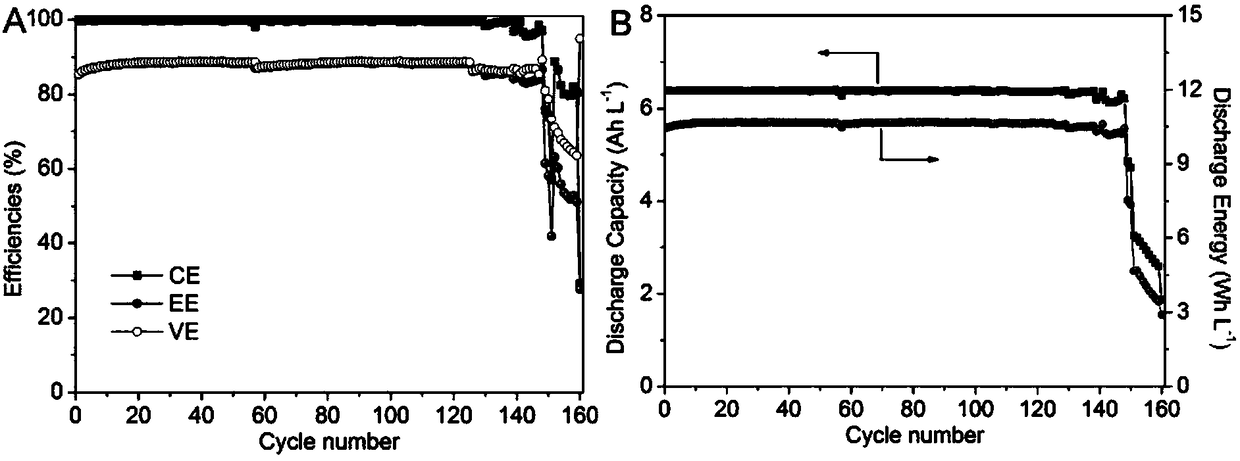
[0045] For a symmetrical alkaline zinc-iron flow battery, the solute composition of the positive electrolyte (aqueous solution) is 0.4mol L -1 Potassium ferrocyanide+0.2mol L -1 Zn(OH) 4 2- +3mol L -1 KOH; negative electrode electrolyte (aqueous solution) solute composition is 0.4mol L -1 Potassium ferrocyanide+0.2mol L -1 Zn(OH) 4 2- +3mol L -1 KOH; the positive and negative electrolyte volumes are 100mL each; the positive and negative electrodes are porous carbon felt electrodes, and the graphite plate is used as a current collector; the ion-conducting membrane is a polybenzimidazole ion-conducting membrane; at 80mA cm -2 Charge for 12min under the condition of the current density, and then cut off the voltage as the condition, 80mA cm -2 Discharge to 0.1V under the condition of current density. At the end of the discharge, measure the volume of the positive and negative electrolytes, from figure 2 It can be seen that within 600 cycles of this symmetrical alkaline...
Embodiment 2
[0050] For a symmetrical alkaline zinc-iron flow battery, the solute composition of the positive electrolyte (aqueous solution) is 0.2mol L -1 Potassium ferrocyanide+0.1mol L -1 Zn(OH) 4 2- +1mol L -1 KOH; negative electrode electrolyte (aqueous solution) solute composition is 0.4mol L -1 Potassium ferrocyanide+0.1mol L -1 Zn(OH) 4 2- +1mol L -1 KOH; the positive and negative electrolyte volumes are 100mL each; the positive and negative electrodes are porous carbon felt electrodes, and the graphite plate is used as a current collector; the ion-conducting membrane is a polybenzimidazole ion-conducting membrane; at 100mAcm -2 Charge for 10min under the condition of the current density, and then cut off the voltage as the condition, 100mA cm -2 Discharge to 0.1V under the condition of current density. Measure the volume of positive and negative electrolyte at the end of discharge. After 481 cycles, the volume of negative electrolyte is 95mL, and the volume of positive el...
Embodiment 3
[0054] For a symmetrical alkaline zinc-iron flow battery, the solute composition of the positive electrolyte (aqueous solution) is 0.6mol L -1 Potassium ferrocyanide+0.3mol L -1 Zn(OH) 4 2- +3mol L -1 KOH; negative electrode electrolyte (aqueous solution) solute composition is 0.6mol L -1 Potassium ferrocyanide+0.3mol L -1 Zn(OH) 4 2- +3mol L -1 KOH; the positive and negative electrolyte volumes are 100mL each; the positive and negative electrodes are porous carbon felt electrodes, and the graphite plate is used as a current collector; the ion-conducting membrane is a polybenzimidazole ion-conducting membrane; at 80mA cm -2 Charge for 15min under the current density condition, and then cut off the voltage as the condition, 80mA cm -2 Discharge to 0.1V under the condition of current density. Measure the volume of the positive and negative electrolytes at the end of discharge. After 527 cycles, the volume of the negative electrolyte is 92mL, and the volume of the positi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com