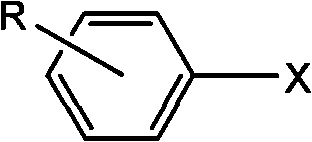
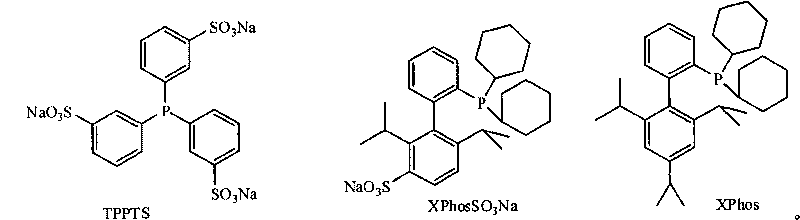
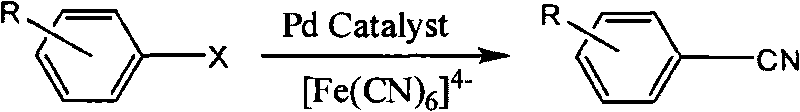Synthetic method of aryl cyanide in water solution
A synthesis method and cyanide technology, which are used in the preparation of cyanide reactions, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of harsh reaction conditions, damage, functional groups cannot be retained, etc., and achieve easy separation and reduce environmental protection. Pollution problems, the effect of reducing the use of organic solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of benzonitrile
[0023] 1.1 Add 2ml of water and 1,4-dioxane to a 25ml Schlenk test tube, the mixture (volume ratio 1:1), then add chlorobenzene (1mmol, 102μL), K 4 [Fe(CN) 6 ] (0.25mmol, 92.1mg), K 2 CO 3 (0.25mmol, 34.5mg), XPhosSO 3 Na(2%mol, 10.5mg), Pd(OAc) 2 (1%mol, 2.2mg), reacted at 110°C for 10h, and the yield of benzonitrile was 93.6% (determined by gas chromatography).
[0024]
[0025] 1.2 Add 2ml of water and 1,4-dioxane to a 25ml Schlenk test tube, the mixture (volume ratio 1.5:0.5), then add chlorobenzene (1mmol, 102μL), K 4 [Fe(CN) 6 ] (0.20mmol, 73.7mg), K 2 CO 3 (0.25mmol, 34.5mg), XPhosSO 3 Na(2%mol, 10.5mg), Pd(OAc) 2 (1%mol, 2.2mg), reacted at 110°C for 10h, and the yield of benzonitrile was 83.2% (determined by gas chromatography).
[0026] 1.3 Add 2ml of water and 1,4-dioxane to a 25ml Schlenk test tube, the mixed solution (volume ratio 1.2:0.8), then add chlorobenzene (1mmol, 102μL), K 4 [Fe(CN) 6 ] (0...
Embodiment 2
[0031] Embodiment 2: the synthesis of 2-methylbenzonitrile
[0032] 2.1 Add 2ml of water and 1,4-dioxane mixture (volume ratio 1:1) to a 25ml Schlenk test tube, then add o-chlorotoluene (1mmol, 117μL), K 4 [Fe(CN) 6 ] (0.25mmol, 92.1mg), K 2 CO 3 (0.25mmol, 34.5mg), XPhosSO 3 Na(3%mol, 15.7mg), Pd(OAc) 2 (1.5%mol, 3.3mg), reacted at 120°C for 10h, and the yield of 2-methylbenzonitrile was 97% (determined by gas chromatography).
[0033]
[0034] 2.2 Add 2ml of water and N,N-dimethylformamide to a 25ml Schlenk test tube to form a mixed solution (volume ratio 1.5:0.5), then add o-chlorotoluene (1mmol, 117μL), K 4 [Fe(CN) 6 ] (0.25mmol, 92.1mg), K 2 CO 3 (0.25mmol, 34.5mg), XPhosSO 3 Na(3%mol, 15.7mg), Pd(OAc) 2 (1.5%mol, 3.3mg), reacted at 120°C for 10h, and the yield of 2-methylbenzonitrile was 60% (determined by gas chromatography).
[0035] 2.3 Add 2ml of water and 1,4-dioxane mixture (volume ratio 1:1) to a 25ml Schlenk test tube, then add o-tolyl-4-methylbenze...
Embodiment 3
[0037] Embodiment 3: the synthesis of 4-methoxybenzonitrile
[0038] 3.1 Add 2ml of water and 1,4-dioxane mixture (volume ratio 1:1) to a 25ml Schlenk test tube, then add 4-chloroanisole (1mmol, 123μL), K 4 [Fe(CN) 6 ] (0.25mmol, 92.1mg), K 2 CO 3 (0.25mmol, 34.5mg), XPhosSO 3 Na(3%mol, 15.7mg), Pd(OAc) 2 (1.5%mol, 3.3mg), reacted at 120°C for 10h, and the yield of 4-methoxybenzonitrile was 96% (determined by gas chromatography).
[0039]
[0040] 3.2 Add 2ml of water and ethanol (volume ratio 1.5:0.5) to a 25ml Schlenk test tube, then add 4-chloroanisole (1mmol, 123μL), K 4 [Fe(CN) 6 ] (0.5mmol, 184.2mg), K 2 CO 3 (0.5mmol, 69.0mg), XPhosSO 3 Na(2%mol, 10.5mg), Pd(OAc) 2 (0.5% mol, 1.1 mg), reacted at 120°C for 10 h, and the yield of 4-methoxybenzonitrile was 45.1% (determined by gas chromatography).
[0041] 3.3 Add 2ml of water and 1.0mmol of tetrabutylammonium bromide mixture to a 25ml Schlenk test tube, then add 4-bromoanisole (1mmol, 125μL), K 4 [Fe(CN) 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com